A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats
- PMID: 22676851
- PMCID: PMC4854615
- DOI: 10.1089/neu.2012.2408
A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats
Abstract
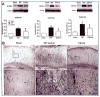
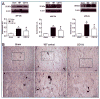
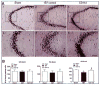
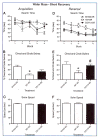
Traumatic brain injury (TBI) is an international health concern often resulting in chronic neurological abnormalities, including cognitive deficits, emotional disturbances, and motor impairments. An anti-CD11d monoclonal antibody that blocks the CD11d/CD18 integrin and vascular cell adhesion molecule (VCAM)-1 interaction following experimental spinal cord injury improves functional recovery, while reducing the intraspinal number of neutrophils and macrophages, oxidative activity, and tissue damage. Since the mechanisms of secondary injury in the brain and spinal cord are similar, we designed a study to evaluate fully the effects of anti-CD11d treatment after a moderate lateral fluid percussion TBI in the rat. Rats were treated at 2 h after TBI with either the anti-CD11d antibody or an isotype-matched control antibody 1B7, and both short (24- to 72-h) and long (4-week) recovery periods were examined. The anti-CD11d integrin treatment reduced neutrophil and macrophage levels in the injured brain, with concomitant reductions in lipid peroxidation, astrocyte activation, amyloid precursor protein accumulation, and neuronal loss. The reduced neuroinflammation seen in anti-CD11d-treated rats correlated with improved performance on a number of behavioral tests. At 24 h, the anti-CD11d group performed significantly better than the 1B7 controls on several water maze measures of spatial cognition. At 4 weeks post-injury the anti-CD11d-treated rats had better sensorimotor function as assessed by the beam task, and reduced anxiety-like behaviors, as evidenced by elevated-plus maze testing, compared to 1B7 controls. These findings suggest that neuroinflammation is associated with behavioral deficits after TBI, and that anti-CD11d antibody treatment is a viable strategy to improve neurological outcomes after TBI.
Figures







Similar articles
-
Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion.J Neuroinflammation. 2013 Feb 15;10:26. doi: 10.1186/1742-2094-10-26. J Neuroinflammation. 2013. PMID: 23414334 Free PMC article.
-
Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats.Brain Res. 2008 May 1;1207:155-63. doi: 10.1016/j.brainres.2008.02.057. Epub 2008 Mar 5. Brain Res. 2008. PMID: 18374312 Free PMC article.
-
CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats.Exp Neurol. 2015 Sep;271:409-22. doi: 10.1016/j.expneurol.2015.07.003. Epub 2015 Jul 11. Exp Neurol. 2015. PMID: 26169930 Free PMC article.
-
Anti-CD11d monoclonal antibody treatment for rat spinal cord compression injury.Exp Neurol. 2012 Feb;233(2):606-11. doi: 10.1016/j.expneurol.2010.11.015. Epub 2010 Dec 9. Exp Neurol. 2012. PMID: 21145887 Free PMC article.
-
β2 Integrin CD11d/CD18: From Expression to an Emerging Role in Staged Leukocyte Migration.Front Immunol. 2021 Nov 8;12:775447. doi: 10.3389/fimmu.2021.775447. eCollection 2021. Front Immunol. 2021. PMID: 34858434 Free PMC article. Review.
Cited by
-
Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion.J Neuroinflammation. 2013 Feb 15;10:26. doi: 10.1186/1742-2094-10-26. J Neuroinflammation. 2013. PMID: 23414334 Free PMC article.
-
Temporal proteomics of human cerebrospinal fluid after severe traumatic brain injury.J Neuroinflammation. 2022 Dec 8;19(1):291. doi: 10.1186/s12974-022-02654-0. J Neuroinflammation. 2022. PMID: 36482407 Free PMC article.
-
Enduring Neuroprotective Effect of Subacute Neural Stem Cell Transplantation After Penetrating TBI.Front Neurol. 2019 Jan 17;9:1097. doi: 10.3389/fneur.2018.01097. eCollection 2018. Front Neurol. 2019. PMID: 30719019 Free PMC article. Review.
-
Chronic Histopathological and Behavioral Outcomes of Experimental Traumatic Brain Injury in Adult Male Animals.J Neurotrauma. 2015 Dec 1;32(23):1861-82. doi: 10.1089/neu.2014.3680. Epub 2015 Apr 15. J Neurotrauma. 2015. PMID: 25490251 Free PMC article. Review.
-
Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma.J Cereb Blood Flow Metab. 2015 Aug;35(8):1339-47. doi: 10.1038/jcbfm.2015.56. Epub 2015 Apr 8. J Cereb Blood Flow Metab. 2015. PMID: 25853909 Free PMC article.
References
-
- Bao F, Chen Y, Dekaban GA, Weaver LC. An anti-CD11d integrin antibody reduces cyclooxygenase-2 expression and protein and DNA oxidation after spinal cord injury in rats. J Neurochem. 2004a;90:1194–1204. - PubMed
-
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004b;88:1335–1344. - PubMed
-
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. - PubMed
This article has been cited by
-
- Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Experimental Neurology. 2014;258:112–120. - PubMed
-
- Shultz Sandy R, TanXin L, Wright David K, Liu Shijie J, Semple Bridgette D, Leigh Johnston, Jones Nigel C, Cook Andrew D, Hamilton John A, O’Brien Terence J. Granulocyte-Macrophage Colony-Stimulating Factor Is Neuroprotective in Experimental Traumatic Brain Injury. Journal of Neurotrauma. 2014;31:10. - PMC - PubMed
-
- John C. GenselSpinal Cord Injury. :339–361.
-
- Gold Eric M, Su Diane, López-Velázquez Luci, Haus Daniel L, Perez Harvey, Lacuesta George A, Anderson Aileen J, Cummings Brian J. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regenerative Medicine. 2013;8:4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

