Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject™ by peripheral health care providers at home births: design of a community-based cluster-randomized trial
- PMID: 22676921
- PMCID: PMC3406972
- DOI: 10.1186/1471-2393-12-42
Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject™ by peripheral health care providers at home births: design of a community-based cluster-randomized trial
Abstract
Background: Hemorrhage is the leading direct cause of maternal death globally. While oxytocin is the drug of choice for postpartum hemorrhage prevention, its use has generally been limited to health facilities. This trial assesses the effectiveness, safety, and feasibility of expanding the use of prophylactic intramuscular oxytocin to peripheral health care providers at home births in four predominantly rural districts in central Ghana.
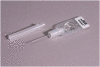
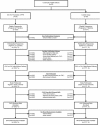
Methods: This study is designed as a community-based cluster-randomized trial in which Community Health Officers are randomized to provide (or not provide) an injection of oxytocin 10 IU via the Uniject™ injection system within one minute of delivery of the baby to women who request their presence at home at the onset of labor. The primary aim is to determine if administration of prophylactic oxytocin via Uniject™ by this cadre will reduce the risk of postpartum hemorrhage by 50 % relative to deliveries which do not receive the prophylactic intervention. Postpartum hemorrhage is examined under three sequential definitions: 1) blood loss ≥500 ml (BL); 2) treatment for bleeding (TX) and/or BL; 3) hospital referral for bleeding and/or TX and/or BL. Secondary outcomes address safety and feasibility of the intervention and include adverse maternal and fetal outcomes and logistical concerns regarding assistance at home births and the storage and handling of oxytocin, respectively.
Discussion: Results from this trial will build evidence for the effectiveness of expanding the delivery of this established prophylactic intervention to peripheral settings. Complementary data on safety and logistical issues related to this intervention will assist policymakers in low-income countries in selecting both the best uterotonic and service delivery strategy for postpartum hemorrhage prevention. Results of this trial are expected in mid-2013. The trial is registered at ClinicalTrials.gov: NCT01108289.
Figures



References
-
- Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database Syst Rev. 2000;2:000007. - PubMed
-
- WHO. WHO Recommendations for the Prevention of Postpartum Haemorrhage. 2007.
-
- FIGO, ICM. Joint Statement. The Hague, London; 2003.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

