IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis
- PMID: 22677045
- PMCID: PMC3470466
- DOI: 10.1016/j.jaci.2012.04.024
IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis
Abstract
Background: In subjects with psoriasis, inflammation and epidermal hyperplasia are thought to be controlled by T cell-derived cytokines. Evidence suggests that the T(H)17 cell cytokine IL-17A (IL-17) might play a role in disease pathogenesis.
Objective: We sought to understand the effect that neutralization of IL-17 has on the clinical features of psoriasis and to understand the role that IL-17 has in inflammatory pathways underlying psoriasis in human subjects.
Methods: We examined skin lesions obtained from 40 subjects participating in a phase I, randomized, double-blind, placebo-controlled trial of the anti-IL-17 mAb ixekizumab (previously LY2439821) in which subjects received 5, 15, 50, or 150 mg of subcutaneous ixekizumab or placebo at weeks 0, 2, and 4.
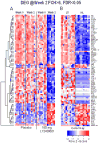
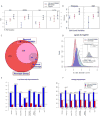
Results: There were significant dose-dependent reductions from baseline in keratinocyte proliferation, hyperplasia, epidermal thickness, infiltration into the dermis and epidermis by T cells and dendritic cells, and keratinocyte expression of innate defense peptides at 2 weeks. By week 6, the skin appeared normal. Quantitative RT-PCR and microarrays revealed an ablation of the disease-defining mRNA expression profile by 2 weeks after the first dose of study drug. The effect of IL-17 blockade on expression of genes synergistically regulated by IL-17 and TNF-α was of higher magnitude at 2 weeks than in prior studies with TNF-α antagonism.
Conclusion: Our data suggest that IL-17 is a key "driver" cytokine that activates pathogenic inflammation in subjects with psoriasis. Neutralizing IL-17 with ixekizumab might be a successful therapeutic strategy in psoriasis.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures

References
-
- Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995 Mar 2;332(9):581–8. - PubMed
-
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007 Feb 22;445(7130):866–73. - PubMed
-
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008 May;128(5):1207–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

