Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy
- PMID: 22677046
- PMCID: PMC4161455
- DOI: 10.1016/j.jaci.2012.04.021
Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy
Erratum in
- J Allergy Clin Immunol. 2012 Nov;130(5):1224
Abstract
Background: Allergen-specific immunotherapy is the only mode of therapy that has been demonstrated to offer a cure in patients with IgE-mediated respiratory allergies.
Objective: We sought to demonstrate the safety and efficacy of timothy grass (TG) and dust mite (DM) dual sublingual immunotherapy (SLIT) and to begin to investigate the immune mechanisms involved in successful immunotherapy with multiple allergens.
Methods: The safety and efficacy of dual SLIT with TG and DM in children and adults with demonstrated allergies to TG and DM were investigated in a single-center, randomized, double-blind, controlled phase I study. Thirty subjects received either TG and DM dual SLIT (n= 20) or placebo (n = 10). Immune parameters were evaluated for differentiation of desensitized subjects from control subjects.
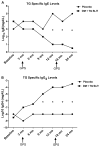
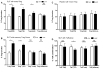
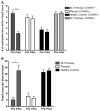
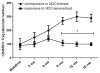
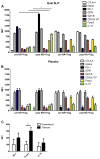
Results: Subjects treated with dual SLIT had decreased rhinoconjunctivitis scores (P < .001) and medication use scores (P < .001) and reduced responses to TG and DM allergen based on results of skin prick tests or nasal disk challenges (P < .01 and P < .001, respectively) compared with placebo-treated control subjects. An increase in TG- and DM-specific IgG(4) levels, reduced allergen-specific IgE levels, and subsequent basophil activation were observed in the active treatment group. Dual SLIT promoted allergen-specific suppressive CD4(+)CD25(high)CD127(low)CD45RO(+) forkhead box protein 3 (Foxp3)(+) memory regulatory T cells with reduced DNA methylation of CpG sites within the Foxp3 locus.
Conclusion: The results of this pilot study suggest that dual SLIT could be an effective means to treat subjects with sensitivities to a variety of allergens and that long-term tolerance might be induced by epigenetic modifications of Foxp3 in memory regulatory T cells.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures

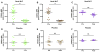
Similar articles
-
Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells.Clin Exp Allergy. 2010 Apr;40(4):598-606. doi: 10.1111/j.1365-2222.2010.03462.x. Epub 2010 Feb 22. Clin Exp Allergy. 2010. PMID: 20184605 Clinical Trial.
-
Intradermal grass pollen immunotherapy increases TH2 and IgE responses and worsens respiratory allergic symptoms.J Allergy Clin Immunol. 2017 Jun;139(6):1830-1839.e13. doi: 10.1016/j.jaci.2016.09.024. Epub 2016 Oct 20. J Allergy Clin Immunol. 2017. PMID: 27773851 Free PMC article. Clinical Trial.
-
Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation.J Allergy Clin Immunol. 2014 Jan;133(1):130-8.e1-2. doi: 10.1016/j.jaci.2013.09.043. Epub 2013 Nov 28. J Allergy Clin Immunol. 2014. PMID: 24290282 Clinical Trial.
-
A meta-analysis of sublingual allergen immunotherapy and pharmacotherapy in pollen-induced seasonal allergic rhinoconjunctivitis.BMC Med. 2014 May 1;12:71. doi: 10.1186/1741-7015-12-71. BMC Med. 2014. PMID: 24885894 Free PMC article. Review.
-
[Specific immunotherapy using allergens apropos of specific immunotherapy by the sublingual route].Allerg Immunol (Paris). 1998 Sep;30(7):221-8. Allerg Immunol (Paris). 1998. PMID: 9823421 Review. French.
Cited by
-
New directions in immunotherapy.Curr Allergy Asthma Rep. 2013 Apr;13(2):178-95. doi: 10.1007/s11882-012-0335-7. Curr Allergy Asthma Rep. 2013. PMID: 23315329 Review.
-
Biomarkers of AIT: Models of prediction of efficacy.Allergol Select. 2022 Nov 21;6:267-275. doi: 10.5414/ALX02333E. eCollection 2022. Allergol Select. 2022. PMID: 36457722 Free PMC article. Review.
-
The Use of Biomarkers to Predict Aero-Allergen and Food Immunotherapy Responses.Clin Rev Allergy Immunol. 2018 Oct;55(2):190-204. doi: 10.1007/s12016-018-8678-z. Clin Rev Allergy Immunol. 2018. PMID: 29455358 Free PMC article. Review.
-
Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut-sensitized mice.Allergy. 2019 Jan;74(1):152-164. doi: 10.1111/all.13479. Epub 2018 Oct 8. Allergy. 2019. PMID: 29779209 Free PMC article.
-
Regulatory T cells and their roles in immune dysregulation and allergy.Immunol Res. 2014 May;58(2-3):358-68. doi: 10.1007/s12026-014-8512-5. Immunol Res. 2014. PMID: 24781194 Free PMC article. Review.
References
-
- Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–29. - PubMed
-
- Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64(suppl 91):1–59. - PubMed
-
- Durham SR, Leung DY. One hundred years of allergen immunotherapy: time to ring the changes. J Allergy Clin Immunol. 2011;127:3–7. - PubMed
-
- Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011;66:725–32. - PubMed
-
- Nelson HS. Subcutaneous immunotherapy. J Allergy Clin Immunol. 2011;128:907, e3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

