Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing
- PMID: 22677073
- PMCID: PMC3498431
- DOI: 10.1016/j.mayocp.2012.02.017
Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing
Abstract
Objective: To perform long QT syndrome and catecholaminergic polymorphic ventricular tachycardia cardiac channel postmortem genetic testing (molecular autopsy) for a large cohort of cases of autopsy-negative sudden unexplained death (SUD).
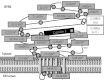
Methods: From September 1, 1998, through October 31, 2010, 173 cases of SUD (106 males; mean ± SD age, 18.4 ± 12.9 years; age range, 1-69 years; 89% white) were referred by medical examiners or coroners for a cardiac channel molecular autopsy. Using polymerase chain reaction, denaturing high-performance liquid chromatography, and DNA sequencing, a comprehensive mutational analysis of the long QT syndrome susceptibility genes (KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2) and a targeted analysis of the catecholaminergic polymorphic ventricular tachycardia type 1-associated gene (RYR2) were conducted.
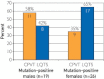
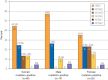
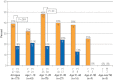
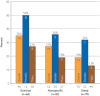
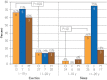
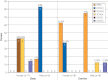
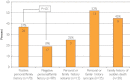
Results: Overall, 45 putative pathogenic mutations absent in 400 to 700 controls were identified in 45 autopsy-negative SUD cases (26.0%). Females had a higher yield (26/67 [38.8%]) than males (19/106 [17.9%]; P<.005). Among SUD cases with exercise-induced death, the yield trended higher among the 1- to 10-year-olds (8/12 [66.7%]) compared with the 11- to 20-year-olds (4/27 [14.8%]; P=.002). In contrast, for those who died during a period of sleep, the 11- to 20-year-olds had a higher yield (9/25 [36.0%]) than the 1- to 10-year-olds (1/24 [4.2%]; P=.01).
Conclusion: Cardiac channel molecular autopsy should be considered in the evaluation of autopsy-negative SUD. Several interesting genotype-phenotype observations may provide insight into the expected yields of postmortem genetic testing for SUD and assist in selecting cases with the greatest potential for mutation discovery and directing genetic testing efforts.
Copyright © 2012 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Virmani R., Burke A.P., Farb A. Sudden cardiac death. Cardiovasc Pathol. 2001;10(6):275–282. - PubMed
-
- Liberthson R.R. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334(16):1039–1044. - PubMed
-
- Chugh S.S., Kelly K.L., Titus J.L. Sudden cardiac death with apparently normal heart. Circulation. 2000;102(6):649–654. - PubMed
-
- Maron B.J., Shirani J., Poliac L.C., Mathenge R., Roberts W.C., Mueller F.O. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276(3):199–204. [see comment] - PubMed
-
- Corrado D., Basso C., Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50(2):399–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

