Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis
- PMID: 22677229
- PMCID: PMC3433241
- DOI: 10.1016/j.leukres.2012.04.022
Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis
Abstract
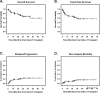
We report a consecutive series of 59 patients with MDS who underwent reduced-intensity hematopoietic stem cell transplantation (RI-HSCT) with fludarabine/melphalan conditioning and tacrolimus/sirolimus-based GVHD prophylaxis. Two-year OS, EFS, and relapse incidences were 75.1%, 65.2%, and 20.9%, respectively. The cumulative incidence of non-relapse mortality at 100 days, 1 year, and 2 years was 3.4%, 8.5%, and 10.5%, respectively. The incidence of grade II-IV acute GVHD was 35.4%; grade III-IV was 18.6%. Forty of 55 evaluable patients developed chronic GVHD; of these 35 were extensive grade. This RI-HSCT protocol produces encouraging outcomes in MDS patients, and tacrolimus/sirolimus-based GVHD prophylaxis may contribute to that promising result.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens.Blood. 2010 Feb 4;115(5):1098-105. doi: 10.1182/blood-2009-03-207563. Epub 2009 Nov 19. Blood. 2010. PMID: 19965688 Free PMC article. Clinical Trial.
-
Outcomes of Patients with Recurrent and Refractory Lymphoma Undergoing Allogeneic Hematopoietic Cell Transplantation with BEAM Conditioning and Sirolimus- and Tacrolimus-Based GVHD Prophylaxis.Biol Blood Marrow Transplant. 2019 Feb;25(2):287-292. doi: 10.1016/j.bbmt.2018.09.009. Epub 2018 Sep 15. Biol Blood Marrow Transplant. 2019. PMID: 30227232 Free PMC article. Clinical Trial.
-
Sirolimus, tacrolimus and low-dose methotrexate based graft-versus-host disease prophylaxis after non-ablative or reduced intensity conditioning in related and unrelated donor allogeneic hematopoietic cell transplant.Leuk Lymphoma. 2015 Mar;56(3):663-70. doi: 10.3109/10428194.2014.930851. Epub 2014 Aug 6. Leuk Lymphoma. 2015. PMID: 24913499 Free PMC article.
-
Busulfan dose intensity and outcomes in reduced-intensity allogeneic peripheral blood stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia.Biol Blood Marrow Transplant. 2013 Jun;19(6):981-7. doi: 10.1016/j.bbmt.2013.03.016. Epub 2013 Apr 2. Biol Blood Marrow Transplant. 2013. PMID: 23562738
-
Hematopoietic stem cell transplantation for MDS.Hematol Oncol Clin North Am. 2010 Apr;24(2):407-22. doi: 10.1016/j.hoc.2010.02.003. Hematol Oncol Clin North Am. 2010. PMID: 20359634 Free PMC article. Review.
Cited by
-
Myelodysplasia: new approaches.Curr Treat Options Oncol. 2013 Jun;14(2):156-69. doi: 10.1007/s11864-013-0224-x. Curr Treat Options Oncol. 2013. PMID: 23436197
-
Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: a multicentre, randomised, phase 3 trial.Lancet Haematol. 2019 Aug;6(8):e409-e418. doi: 10.1016/S2352-3026(19)30088-2. Epub 2019 Jun 24. Lancet Haematol. 2019. PMID: 31248843 Free PMC article. Clinical Trial.
-
Glomerular diseases post-hematopoietic stem cell transplantation: pathologic spectrum and plausible mechanisms.Clin Kidney J. 2023 Feb 6;16(6):896-900. doi: 10.1093/ckj/sfad023. eCollection 2023 Jun. Clin Kidney J. 2023. PMID: 37261003 Free PMC article.
-
Precision sirolimus dosing in children: The potential for model-informed dosing and novel drug monitoring.Front Pharmacol. 2023 Mar 20;14:1126981. doi: 10.3389/fphar.2023.1126981. eCollection 2023. Front Pharmacol. 2023. PMID: 37021042 Free PMC article. Review.
-
Decision Analysis of Transplantation for Patients with Myelodysplasia: "Who Should We Transplant Today?".Curr Hematol Malig Rep. 2020 Aug;15(4):305-315. doi: 10.1007/s11899-020-00573-6. Curr Hematol Malig Rep. 2020. PMID: 32222884 Free PMC article. Review.
References
-
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. - PubMed
-
- Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. - PubMed
-
- Anderson JE, Appelbaum FR, Fisher LD, et al. Allogeneic bone marrow transplantation for 93 patients with myelodysplastic syndrome. Blood. 1993;82:677–681. - PubMed
-
- Zikos P, Van Lint MT, Frassoni F, et al. Low transplant mortality in allogeneic bone marrow transplantation for acute myeloid leukemia: a randomized study of low-dose cyclosporin versus low-dose cyclosporin and low-dose methotrexate. Blood. 1998;91:3503–3508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

