Haloenol pyranones and morpholinones as antineoplastic agents of prostate cancer
- PMID: 22677312
- PMCID: PMC3376906
- DOI: 10.1016/j.bmcl.2012.05.038
Haloenol pyranones and morpholinones as antineoplastic agents of prostate cancer
Abstract
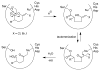
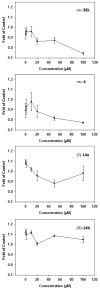
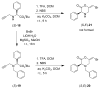
Haloenol pyran-2-ones and morpholin-2-ones were synthesized and evaluated as inhibitors of cell growth in two different prostate human cancer cell lines (PC-3 and LNCaP). Analogs derived from L- and D-phenylglycine were found to be the most effective antagonists of LNCaP and PC-3 cell growth. Additional studies reveal that the inhibitors induced G2/M arrest and the (S)-enantiomer of the phenylglycine-based derivatives was a more potent inhibitor of cytosolic iPLA(2)β.
Published by Elsevier Ltd.
Figures
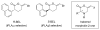



Similar articles
-
Pyranone, thiopyranone, and pyridone inhibitors of phosphatidylinositol 3-kinase related kinases. Structure-activity relationships for DNA-dependent protein kinase inhibition, and identification of the first potent and selective inhibitor of the ataxia telangiectasia mutated kinase.J Med Chem. 2007 Apr 19;50(8):1958-72. doi: 10.1021/jm061121y. Epub 2007 Mar 20. J Med Chem. 2007. PMID: 17371003
-
New Approaches for the Synthesis of Fused Thiophene, Pyrazole, Pyran and Pyridine Derivatives with Anti-Proliferative Together with c-Met Kinase and Prostate Cancer Cell Inhibitions.Anticancer Agents Med Chem. 2021;21(13):1751-1766. doi: 10.2174/1871520620999201110191056. Anticancer Agents Med Chem. 2021. PMID: 33176664
-
20-Cyclopropyl-cholecalciferol vitamin D3 analogs: a unique class of potent inhibitors of proliferation of human prostate, breast and myeloid leukemia cell lines.Anticancer Res. 1999 May-Jun;19(3A):1689-97. Anticancer Res. 1999. PMID: 10470102
-
Phthalocyanine derivatives possessing 2-(morpholin-4-yl)ethoxy groups as potential agents for photodynamic therapy.J Med Chem. 2015 Mar 12;58(5):2240-55. doi: 10.1021/acs.jmedchem.5b00052. Epub 2015 Mar 3. J Med Chem. 2015. PMID: 25700089
-
Inhibition of proliferation of prostate cancer cells by a 19-nor-hexafluoride vitamin D3 analogue involves the induction of p21waf1, p27kip1 and E-cadherin.J Mol Endocrinol. 1997 Aug;19(1):15-27. doi: 10.1677/jme.0.0190015. J Mol Endocrinol. 1997. PMID: 9278857
Cited by
-
Towards lipidomics of low-abundant species for exploring tumor heterogeneity guided by high-resolution mass spectrometry imaging.Int J Mol Sci. 2013 Dec 17;14(12):24560-80. doi: 10.3390/ijms141224560. Int J Mol Sci. 2013. PMID: 24351834 Free PMC article.
References
-
- Rai R, Katzenellenbogen JA. J Med Chem. 1992;35:4150. - PubMed
- Reed PE, Katzenellenbogen JA. J Biol Chem. 1991;266:13. - PubMed
- Sofia MJ, Katzenellenbogen JA. J Med Chem. 1986;29:230. - PubMed
- Daniels SB, Katzenellenbogen JA. Biochemistry. 1986;25:1436. - PubMed
- Daniels SB, Cooney E, Sofia MJ, Chakravarty PK, Katzenellenbogen JA. J Biol Chem. 1983;258:15046. - PubMed
- Chakravarty PK, Krafft GA, Katzenellenbogen JA. J Biol Chem. 1982;257:610. - PubMed
- Krafft GA, Katzenellenbogen JA. J Am Chem Soc. 1981;103:5459.
- Rando RR. Science. 1974;185:320. - PubMed
-
- McHowat J, Creer MH. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:209. - PubMed
-
- Song K, Zhang X, Zhao CY, Ang NT, Ma ZMA. Mol Endocrinol. 2005;19:504. - PMC - PubMed
- Ramanadham S, Song HW, Hsu FF, Zhang S, Crankshaw M, Grant GA, Newgard CB, Bao SZ, Ma ZM, Turk J. Biochemistry. 2003;42:13929. - PMC - PubMed
- Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. J Biol Chem. 1999;274:9607. - PMC - PubMed
- Ma Z, Ramanadham S, Hu Z, Turk J. Biochim Biophys Acta. 1998;1391:384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

