A high-yielding synthesis of allyl glycosides from peracetylated glycosyl donors
- PMID: 22677518
- PMCID: PMC3396721
- DOI: 10.1016/j.carres.2012.05.008
A high-yielding synthesis of allyl glycosides from peracetylated glycosyl donors
Erratum in
- Carbohydr Res. 2013 May 24;373:75
Abstract
β-Configured peracetylated sugars are often used as easily accessible glycosyl donors that are typically activated with common Lewis acids such as boron trifluoride or trimethylsilyltrifluoromethane sulfonate. Often these glycosylations occur with unsatisfactory yields due to incomplete reactions or extensive byproduct formation, primarily as a result of loss of an additional acetyl group generating partially unprotected glycosides. Here we report a simple glycosylation-reacetylation protocol for the generation of predominantly β-configured peracetylated allyl glucoside, -galactoside, -lactoside, and -maltoside with substantially improved reaction yields.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
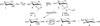

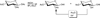
Similar articles
-
Efficient synthesis of omega-mercaptoalkyl 1,2-trans-glycosides from sugar peracetates.Carbohydr Res. 2007 Jun 11;342(8):1009-20. doi: 10.1016/j.carres.2007.02.024. Epub 2007 Feb 25. Carbohydr Res. 2007. PMID: 17362892
-
Glycosyl trichloroacetylcarbamate: a new glycosyl donor for O-glycosylation.Carbohydr Res. 2005 Dec 12;340(17):2688-92. doi: 10.1016/j.carres.2005.07.024. Epub 2005 Oct 5. Carbohydr Res. 2005. PMID: 16212950
-
Variations on the SnCl4 and CF3CO2Ag-promoted glycosidation of sugar acetates: a direct, versatile and apparently simple method with either alpha or beta stereocontrol.Carbohydr Res. 2009 Sep 8;344(13):1646-53. doi: 10.1016/j.carres.2009.06.004. Epub 2009 Jun 6. Carbohydr Res. 2009. PMID: 19596225
-
O-Glycosylation methods in the total synthesis of complex natural glycosides.Nat Prod Rep. 2015 Sep;32(9):1331-55. doi: 10.1039/c5np00033e. Nat Prod Rep. 2015. PMID: 26067865 Review.
-
Glycosyl fluorides in glycosidations.Carbohydr Res. 2000 Jul 10;327(1-2):15-26. doi: 10.1016/s0008-6215(99)00325-0. Carbohydr Res. 2000. PMID: 10968674 Review.
Cited by
-
Synthesis of Galα(1,3)Galβ(1,4)GlcNAcα-, Galβ(1,4)GlcNAcα- and GlcNAc-containing neoglycoproteins and their immunological evaluation in the context of Chagas disease.Glycobiology. 2016 Jan;26(1):39-50. doi: 10.1093/glycob/cwv081. Epub 2015 Sep 18. Glycobiology. 2016. PMID: 26384953 Free PMC article.
-
Maltotriose-based probes for fluorescence and photoacoustic imaging of bacterial infections.Nat Commun. 2020 Mar 6;11(1):1250. doi: 10.1038/s41467-020-14985-8. Nat Commun. 2020. PMID: 32144257 Free PMC article.
References
-
- Gigg R, Payne S, Conat R. Carbohydr. Res. 1983;2:207–223.
-
- Ogawa T, Nakabayashi S. Carbohydr. Res. 1981;93:C1–C5. - PubMed
-
- Eichler E, Kihlberg J, Bundle DR. Glycoconj. J. 1991;8:69–74. - PubMed
-
- Lee RT, Lee YC. Carbohydr. Res. 1974;37:193–201. - PubMed
- Seeventer PBv, Dorst JALMv, Siemerink JF, Kamerling JP, Vliegenthart JFG. Carbohydr. Res. 1997;300:369–373.
-
- Matsuoka K, Oka H, Koyama T, Esumi Y, Terunuma D. Tetrahedron Lett. 2001;42:3327–3330.
- Houseman BT, Gawalt ES, Mrksich M. Langmuir. 2003;19:1522–1531.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

