Deconstructing 14-phenylpropyloxymetopon: minimal requirements for binding to mu opioid receptors
- PMID: 22677527
- PMCID: PMC3401595
- DOI: 10.1016/j.bmc.2012.05.006
Deconstructing 14-phenylpropyloxymetopon: minimal requirements for binding to mu opioid receptors
Abstract
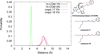
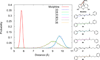
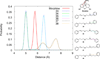
A series of phenylpropyloxyethylamines and cinnamyloxyethylamines were synthesized as deconstructed analogs of 14-phenylpropyloxymetopon and analyzed for opioid receptor binding affinity. Using the Conformationally Sampled Pharmacophore modeling approach, we discovered a series of compounds lacking a tyrosine mimetic, historically considered essential for μ opioid binding. Based on the binding studies, we have identified the optimal analogs to be N-methyl-N-phenylpropyl-2-(3-phenylpropoxy)ethanamine, with 1520 nM, and 2-(cinnamyloxy)-N-methyl-N-phenethylethanamine with 1680 nM affinity for the μ opioid receptor. These partial opioid structure analogs will serve as the novel lead compounds for future optimization studies.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Effect of ring-constrained phenylpropyloxyethylamines on sigma receptors.Bioorg Med Chem. 2013 Sep 1;21(17):4923-7. doi: 10.1016/j.bmc.2013.06.068. Epub 2013 Jul 11. Bioorg Med Chem. 2013. PMID: 23896610 Free PMC article.
-
Molecular modeling studies to predict the possible binding modes of endomorphin analogs in mu opioid receptor.Bioorg Med Chem Lett. 2009 Sep 15;19(18):5387-91. doi: 10.1016/j.bmcl.2009.07.121. Epub 2009 Jul 30. Bioorg Med Chem Lett. 2009. PMID: 19679474
-
Stepwise modulation of neurokinin-3 and neurokinin-2 receptor affinity and selectivity in quinoline tachykinin receptor antagonists.J Med Chem. 2001 May 24;44(11):1675-89. doi: 10.1021/jm000501v. J Med Chem. 2001. PMID: 11356103
-
Opioid peptide receptor studies. 7. The methylfentanyl congener RTI-4614-4 and its four enantiomers bind to different domains of the rat mu opioid receptor.Synapse. 1998 Feb;28(2):117-24. doi: 10.1002/(SICI)1098-2396(199802)28:2<117::AID-SYN2>3.0.CO;2-E. Synapse. 1998. PMID: 9450512
-
14-amino, 14-alkylamino, and 14-acylamino analogs of oxymorphindole. Differential effects on opioid receptor binding and functional profiles.J Med Chem. 2003 Apr 10;46(8):1563-6. doi: 10.1021/jm021073r. J Med Chem. 2003. PMID: 12672258
Cited by
-
Effect of ring-constrained phenylpropyloxyethylamines on sigma receptors.Bioorg Med Chem. 2013 Sep 1;21(17):4923-7. doi: 10.1016/j.bmc.2013.06.068. Epub 2013 Jul 11. Bioorg Med Chem. 2013. PMID: 23896610 Free PMC article.
References
-
- Zieglgansberger W, Tolle TR, Zimprich A, Hollt V, Spanagel R. Pain and the Brain. 1995;22:439.
-
- Stein C, Schafer M, Machelska H. Nat Med. 2003;9:1003. - PubMed
-
- Kieffer BL, Evans CJ. Cell. 2002;108:587. - PubMed
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Pain Physician. 2008;11:S105. - PubMed
-
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, Lau J, Carr D. J Pain. 2003;4:231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

