Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form
- PMID: 22677548
- PMCID: PMC3387659
- DOI: 10.1101/gad.191866.112
Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form
Abstract
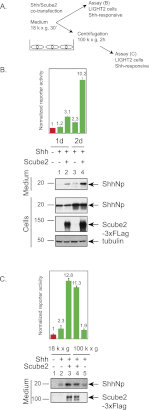
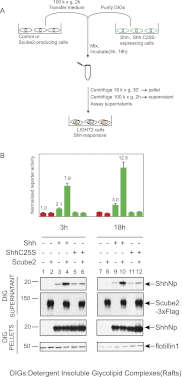
Owing to their covalent modification by cholesterol and palmitate, Hedgehog (Hh) signaling proteins are localized predominantly to the plasma membrane of expressing cells. Yet Hh proteins are also capable of mobilizing to and eliciting direct responses from distant cells. The zebrafish you gene, identified genetically >15 years ago, was more recently shown to encode a secreted glycoprotein that acts cell-nonautonomously in the Hh signaling pathway by an unknown mechanism. We investigated the function of the protein encoded by murine Scube2, an ortholog of you, and found that it mediates release in soluble form of the mature, cholesterol- and palmitate-modified Sonic hedgehog protein signal (ShhNp) when added to cultured cells or purified detergent-resistant membrane microdomains containing ShhNp. The efficiency of Scube2-mediated release of ShhNp is enhanced by the palmitate adduct of ShhNp and by coexpression in ShhNp-producing cells of mDispatchedA (mDispA), a transporter-like protein with a previously defined role in the release of lipid-modified Hh signals. The structural determinants of Scube2 required for its activity in cultured cell assays match those required for rescue of you mutant zebrafish embryos, and we thus conclude that the role of Scube/You proteins in Hh signaling in vivo is to facilitate the release and mobilization of Hh proteins for distant action.
Figures

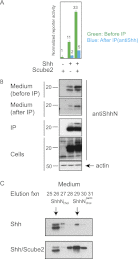
 )
)

Comment in
-
Cell signalling: releasing a hedgehog.Nat Rev Mol Cell Biol. 2012 Aug;13(8):482. doi: 10.1038/nrm3396. Nat Rev Mol Cell Biol. 2012. PMID: 22760119 No abstract available.
-
Zebrafish genetics gets the Scube on Hedgehog secretion.Genes Dev. 2012 Nov 15;26(22):2468-70. doi: 10.1101/gad.207126.112. Genes Dev. 2012. PMID: 23154980 Free PMC article.
References
-
- Beachy PA, Karhadkar SS, Berman DM 2004. Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331 - PubMed
-
- Briscoe J, Chen Y, Jessell TM, Struhl G 2001. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell 7: 1279–1291 - PubMed
-
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K 1999. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99: 803–815 - PubMed
-
- Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, Rakeman AS, Alcorn HL, Anderson KV 2002. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol 12: 1628–1632 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
