Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4
- PMID: 22677551
- PMCID: PMC3402284
- DOI: 10.1681/ASN.2011111077
Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4
Abstract
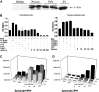
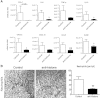
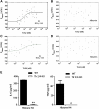
In AKI, dying renal cells release intracellular molecules that stimulate immune cells to secrete proinflammatory cytokines, which trigger leukocyte recruitment and renal inflammation. Whether the release of histones, specifically, from dying cells contributes to the inflammation of AKI is unknown. In this study, we found that dying tubular epithelial cells released histones into the extracellular space, which directly interacted with Toll-like receptor (TLR)-2 (TLR2) and TLR4 to induce MyD88, NF-κB, and mitogen activated protein kinase signaling. Extracellular histones also had directly toxic effects on renal endothelial cells and tubular epithelial cells in vitro. In addition, direct injection of histones into the renal arteries of mice demonstrated that histones induce leukocyte recruitment, microvascular vascular leakage, renal inflammation, and structural features of AKI in a TLR2/TLR4-dependent manner. Antihistone IgG, which neutralizes the immunostimulatory effects of histones, suppressed intrarenal inflammation, neutrophil infiltration, and tubular cell necrosis and improved excretory renal function. In summary, the release of histones from dying cells aggravates AKI via both its direct toxicity to renal cells and its proinflammatory effects. Because the induction of proinflammatory cytokines in dendritic cells requires TLR2 and TLR4, these results support the concept that renal damage triggers an innate immune response, which contributes to the pathogenesis of AKI.
Figures






Comment in
-
Dying cells and extracellular histones in AKI: beyond a NET effect?J Am Soc Nephrol. 2012 Aug;23(8):1275-7. doi: 10.1681/ASN.2012060615. Epub 2012 Jul 12. J Am Soc Nephrol. 2012. PMID: 22797184 Free PMC article. No abstract available.
References
-
- Bonventre JV, Zuk A: Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 - PubMed
-
- Swaminathan S, Griffin MD: First responders: understanding monocyte-lineage traffic in the acutely injured kidney. Kidney Int 74: 1509–1511, 2008 - PubMed
-
- Lichtnekert J, Vielhauer V, Zecher D, Kulkarni OP, Clauss S, Segerer S, Hornung V, Mayadas TN, Beutler B, Akira S, Anders HJ: Trif is not required for immune complex glomerulonephritis: Dying cells activate mesangial cells via Tlr2/Myd88 rather than Tlr3/Trif. Am J Physiol Renal Physiol 296: F867–F874, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

