A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation
- PMID: 22677718
- PMCID: PMC3924965
- DOI: 10.1136/gutjnl-2012-302483
A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation
Abstract
Objectives: Chronic constipation in diabetes mellitus is associated with colonic motor dysfunction and is managed with laxatives. Cholinesterase inhibitors increase colonic motility. This study evaluated the effects of a cholinesterase inhibitor on gastrointestinal and colonic transit and bowel function in diabetic patients with constipation.
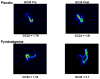
Design: After a 9-day baseline period, 30 patients (mean ± SEM age 50 ± 2 years) with diabetes mellitus (18 type 1, 12 type 2) and chronic constipation without defaecatory disorder were randomised to oral placebo or pyridostigmine, starting with 60 mg three times a day, increasing by 60 mg every third day up to the maximum tolerated dose or 120 mg three times a day; this dose was maintained for 7 days. Gastrointestinal and colonic transit (assessed by scintigraphy) and bowel function were evaluated at baseline and the final 3 and 7 days of treatment, respectively. Treatment effects were compared using analysis of covariance, with gender, body mass index and baseline colonic transit as covariates.
Results: 19 patients (63%) had moderate or severe autonomic dysfunction; 16 (53%) had diabetic retinopathy. 14 of 16 patients randomised to pyridostigmine tolerated 360 mg daily; two patients took 180 mg daily. Compared with placebo (mean ± SEM 1.98 ± 0.17 (baseline), 1.84 ± 0.16 (treatment)), pyridostigmine accelerated (1.96 ± 0.18 (baseline), 2.45 ± 0.2 units (treatment), p<0.01) overall colonic transit at 24 h, but not gastric emptying or small-intestinal transit. Treatment effects on stool frequency, consistency and ease of passage were significant (p ≤ 0.04). Cholinergic side effects were somewhat more common with pyridostigmine (p=0.14) than with placebo.
Conclusions: Cholinesterase inhibition with oral pyridostigmine accelerates colonic transit and improves bowel function in diabetic patients with chronic constipation.
Conflict of interest statement
Figures




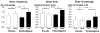
References
-
- Janatuinen E, Pikkarainen P, Laakso M, et al. Gastrointestinal symptoms in middle-aged diabetic patients. Scand J Gastroenterol. 1993;28:427–32. - PubMed
-
- Maleki D, Locke GR, 3rd, Camilleri M, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–16. - PubMed
-
- Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–11. - PubMed
-
- Hasler WL, Coleski R, Chey WD, et al. Differences in intragastric pH in diabetic vs. idiopathic gastroparesis: relation to degree of gastric retention. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1384–91. - PubMed
-
- Buysschaert M, Donckier J, Dive A, et al. Gastric acid and pancreatic polypeptide responses to sham feeding are impaired in diabetic subjects with autonomic neuropathy. Diabetes. 1985;34:1181–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
