Phase II trial of hu14.18-IL2 for patients with metastatic melanoma
- PMID: 22678096
- PMCID: PMC3502633
- DOI: 10.1007/s00262-012-1286-5
Phase II trial of hu14.18-IL2 for patients with metastatic melanoma
Abstract
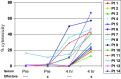
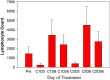
Phase I testing of the hu14.18-IL2 immunocytokine in melanoma patients showed immune activation, reversible toxicities, and a maximal tolerated dose of 7.5 mg/m(2)/day. In this phase II study, 14 patients with measurable metastatic melanoma were scheduled to receive hu14.18-IL2 at 6 mg/m(2)/day as 4-h intravenous infusions on Days 1, 2, and 3 of each 28 day cycle. Patients with stable disease (SD) or regression following cycle 2 could receive two additional treatment cycles. The primary objective was to evaluate antitumor activity and response duration. Secondary objectives evaluated adverse events and immunologic activation. All patients received two cycles of treatment. One patient had a partial response (PR) [1 PR of 14 patients = response rate of 7.1 %; confidence interval, 0.2-33.9 %], and 4 patients had SD and received cycles 3 and 4. The PR and SD responses lasted 3-4 months. All toxicities were reversible and those resulting in dose reduction included grade 3 hypotension (2 patients) and grade 2 renal insufficiency with oliguria (1 patient). Patients had a peripheral blood lymphocytosis on Day 8 and increased C-reactive protein. While one PR in 14 patients met protocol criteria to proceed to stage 2 and enter 16 additional patients, we suspended stage 2 due to limited availability of hu14.18-IL2 at that time and the brief duration of PR and SD. We conclude that subsequent testing of hu14.18-IL2 should involve melanoma patients with minimal residual disease based on compelling preclinical data and the confirmed immune activation with some antitumor activity in this study.
Conflict of interest statement
The authors have the following financial or other conflicts of interests to disclose related to this publication: The commercial rights to hu14.18-IL2 IC are now held by Apeiron-Biologics AG of Vienna Austria. They were licensed from Merck Serono of Darmstadt Germany. A separate (subsequent and ongoing) trial of hu14.18-IL2 in patients with melanoma, chaired by Dr. Mark Albertini and involving this clinical team, received partial per patient research support for components of the clinical study from Merck Serono. No direct support from Merck Serono was received regarding the study reported on here. Dr. Gillies has a patent related to hu14.18-IL2 that he has licensed to Merck Serono.
Figures
Similar articles
-
A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group.Clin Cancer Res. 2006 Mar 15;12(6):1750-9. doi: 10.1158/1078-0432.CCR-05-2000. Clin Cancer Res. 2006. PMID: 16551859 Free PMC article. Clinical Trial.
-
Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients.J Clin Oncol. 2004 Nov 15;22(22):4463-73. doi: 10.1200/JCO.2004.11.035. Epub 2004 Oct 13. J Clin Oncol. 2004. PMID: 15483010 Free PMC article. Clinical Trial.
-
Antitumor Activity and Tolerability of hu14.18-IL2 with GMCSF and Isotretinoin in Recurrent or Refractory Neuroblastoma: A Children's Oncology Group Phase II Study.Clin Cancer Res. 2019 Oct 15;25(20):6044-6051. doi: 10.1158/1078-0432.CCR-19-0798. Epub 2019 Jul 29. Clin Cancer Res. 2019. PMID: 31358541 Free PMC article. Clinical Trial.
-
The development of antibody-IL-2 based immunotherapy with hu14.18-IL2 (EMD-273063) in melanoma and neuroblastoma.Expert Opin Investig Drugs. 2009 Jul;18(7):991-1000. doi: 10.1517/13543780903048911. Expert Opin Investig Drugs. 2009. PMID: 19548853 Free PMC article. Review.
-
High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993.J Clin Oncol. 1999 Jul;17(7):2105-16. doi: 10.1200/JCO.1999.17.7.2105. J Clin Oncol. 1999. PMID: 10561265 Review.
Cited by
-
An engineered immunocytokine with collagen affinity improves the tumor bioavailability, tolerability, and therapeutic efficacy of IL-2.Cell Rep Med. 2023 Nov 21;4(11):101289. doi: 10.1016/j.xcrm.2023.101289. Cell Rep Med. 2023. PMID: 37992685 Free PMC article.
-
Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma.Cancer. 2014 Feb 15;120(4):548-54. doi: 10.1002/cncr.28461. Epub 2013 Oct 25. Cancer. 2014. PMID: 24166473 Free PMC article.
-
Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy.Expert Rev Clin Immunol. 2014 Feb;10(2):207-17. doi: 10.1586/1744666X.2014.875856. Expert Rev Clin Immunol. 2014. PMID: 24410537 Free PMC article. Review.
-
IL-2 based cancer immunotherapies: an evolving paradigm.Front Immunol. 2024 Jul 24;15:1433989. doi: 10.3389/fimmu.2024.1433989. eCollection 2024. Front Immunol. 2024. PMID: 39114660 Free PMC article. Review.
-
Immune Microenvironment in Childhood Cancers: Characteristics and Therapeutic Challenges.Cancers (Basel). 2024 Jun 12;16(12):2201. doi: 10.3390/cancers16122201. Cancers (Basel). 2024. PMID: 38927907 Free PMC article. Review.
References
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. - PubMed
-
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. - PubMed
-
- Harel W, Shau H, Hadley CG, Morgan AC, Jr, Reisfeld RA, Cheresh DA, Mitchell MS. Increased lysis of melanoma by in vivo-elicited human lymphokine-activated killer cells after addition of antiganglioside antibodies in vitro. Cancer Res. 1990;50:6311–6315. - PubMed
-
- Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, Sondel PM. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50:5234–5239. - PubMed
-
- Bajorin DF, Chapman PB, Wong G, Coit DG, Kunicka J, Dimaggio J, Cordon-Cardo C, Urmacher C, Dantes L, Templeton MA, et al. Phase I evaluation of a combination of monoclonal antibody R24 and interleukin 2 in patients with metastatic melanoma. Cancer Res. 1990;50:7490–7495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

