5-(5-Aryl-1,3,4-oxadiazole-2-carbonyl)furan-3-carboxylate and new cyclic C-glycoside analogues from carbohydrate precursors with MAO-B, antimicrobial and antifungal activities
- PMID: 22678415
- PMCID: PMC6268659
- DOI: 10.3390/molecules17067010
5-(5-Aryl-1,3,4-oxadiazole-2-carbonyl)furan-3-carboxylate and new cyclic C-glycoside analogues from carbohydrate precursors with MAO-B, antimicrobial and antifungal activities
Abstract
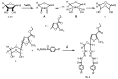
Cyclization of acyclic C-glycoside derivatives 1a,b to 2a,b as the major isomers, and 4a,b as the minor isomers were carried out. The isopropylidene derivatives 3a,b were prepared, as well as the hydrazide derivative 6, which was condensed with a variety of aldehydes to give hydrazones 7a-e which were also prepared from the compounds 12a-e. Acetylation of 7a,d gave the corresponding acetyl derivatives 8a,d, respectively. In addition, the dicarbonyl compound 9 was prepared in the hydrate form, which reacted with a number of aroylhydrazines to give the corresponding bisaroylhydrazones 10a-d, which were cyclized into 1,3,4-oxadiazoles 11a-d. Furthermore, two of the prepared compounds were examined to show the ability to activate MAO-B. In addition a number of prepared compounds showed antibacterial and antiviral activities.
Figures






Similar articles
-
Antimicrobial studies of 2,4-dichloro-5-fluorophenyl containing oxadiazoles.Eur J Med Chem. 2008 Jan;43(1):25-31. doi: 10.1016/j.ejmech.2007.03.013. Epub 2007 Apr 3. Eur J Med Chem. 2008. PMID: 17521777
-
Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents.Acta Pol Pharm. 2010 May-Jun;67(3):247-53. Acta Pol Pharm. 2010. PMID: 20524426
-
Synthesis and evaluation of some new 1,3,4-oxadiazoles bearing thiophene, thiazole, coumarin, pyridine and pyridazine derivatives as antiviral agents.Acta Pharm. 2019 Jun 1;69(2):261-276. doi: 10.2478/acph-2019-0015. Acta Pharm. 2019. PMID: 31259726
-
Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives.Int J Mol Sci. 2021 Jun 29;22(13):6979. doi: 10.3390/ijms22136979. Int J Mol Sci. 2021. PMID: 34209520 Free PMC article. Review.
-
1,3,4-oxadiazole derivatives as potential biological agents.Mini Rev Med Chem. 2013 Oct;13(12):1725-43. doi: 10.2174/13895575113139990071. Mini Rev Med Chem. 2013. PMID: 23815585 Review.
Cited by
-
Antioxidant and antitumor activities of new synthesized aromatic C-nucleoside derivatives.Molecules. 2014 Apr 22;19(4):5163-90. doi: 10.3390/molecules19045163. Molecules. 2014. PMID: 24759075 Free PMC article.
-
Synthesis and Cytotoxic Activity of New Thiazolopyrimidine Sugar Hydrazones and Their Derived Acyclic Nucleoside Analogues.Molecules. 2020 Jan 18;25(2):399. doi: 10.3390/molecules25020399. Molecules. 2020. PMID: 31963649 Free PMC article.
-
Synthesis and bioassay of a new class of furanyl-1,3,4-oxadiazole derivatives.Molecules. 2013 Jul 19;18(7):8550-62. doi: 10.3390/molecules18078550. Molecules. 2013. PMID: 23877049 Free PMC article.
-
A Review on Schiff Base as a Versatile Fluorescent Chemo-sensors Tool for Detection of Cu2+ and Fe3+ Metal Ion.J Fluoresc. 2023 Jul;33(4):1241-1272. doi: 10.1007/s10895-022-03102-1. Epub 2023 Jan 28. J Fluoresc. 2023. PMID: 36708420 Review.
References
-
- Palusa S.K.G., Udupi R.H., Himabindu V., Sridhara A.M. Synthesis and evaluation of a series of pyrimidine substituted 1,3,4-oxadiazole derivatives as antimicrobial and anti-inflammatory agents. Org. Commun. 2011;4:82–93.
-
- Tan T.M.C., Chen Y., Kong K.H., Bai J., Li Y., Lim S.G., Ang T.H., Lam Y. Synthesis and the biological evaluation of 2-benzenesulfonyl alkyl-5-substituted-sulfanyl-(1,3,4)-oxadiazoles as potential anti-hepatitis B virus agents. Antiviral Res. 2006;71:7–14. doi: 10.1016/j.antiviral.2006.02.007. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

