Kynurenines in the mammalian brain: when physiology meets pathology
- PMID: 22678511
- PMCID: PMC3681811
- DOI: 10.1038/nrn3257
Kynurenines in the mammalian brain: when physiology meets pathology
Abstract
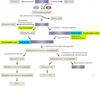
The essential amino acid tryptophan is not only a precursor of serotonin but is also degraded to several other neuroactive compounds, including kynurenic acid, 3-hydroxykynurenine and quinolinic acid. The synthesis of these metabolites is regulated by an enzymatic cascade, known as the kynurenine pathway, that is tightly controlled by the immune system. Dysregulation of this pathway, resulting in hyper-or hypofunction of active metabolites, is associated with neurodegenerative and other neurological disorders, as well as with psychiatric diseases such as depression and schizophrenia. With recently developed pharmacological agents, it is now possible to restore metabolic equilibrium and envisage novel therapeutic interventions.
Figures






References
-
- Liebig J. Über Kynurensäure. Justus Liebig's Ann. Chem. 1853;86:125–126.
-
- Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am. J. Clin. Nutr. 1971;24:659–672. - PubMed
-
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. - PubMed
-
- Parsons CG, et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997;283:1264–1275. - PubMed
-
- Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. Some central effects of kynurenic acid, 7-chlorokynurenic acid and 5,7-dichloro-kynurenic acid, glycine site antagonists. Pol. J. Pharmacol. 1994;46:115–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

