Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artery occlusion stroke models
- PMID: 22678884
- PMCID: PMC3955207
- DOI: 10.1007/s12031-012-9818-1
Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artery occlusion stroke models
Abstract
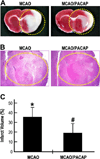
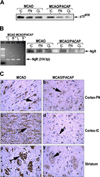
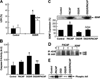
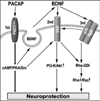
Pituitary adenylate cyclase activating peptide (PACAP), a potent neuropeptide which crosses the blood-brain barrier, is known to provide neuroprotection in rat stroke models of middle cerebral artery occlusion (MCAO) by mechanism(s) which deserve clarification. We confirmed that following i.v. injection of 30 ng/kg of PACAP38 in rats exposed to 2 h of MCAO focal cerebral ischemia and 48 h reoxygenation, 50 % neuroprotection was measured by reduced caspase-3 activity and volume of cerebral infarction. Similar neuroprotective effects were measured upon PACAP38 treatment of oxygen-glucose deprivation and reoxygenation of brain cortical neurons. The neuroprotection was temporally associated with increased expression of brain-derived neurotrophic factor, phosphorylation of its receptor-tropomyosin-related kinase receptor type B (trkB), activation of phosphoinositide 3-kinase and Akt, and reduction of extracellular signal-regulated kinases 1/2 phosphorylation. PACAP38 increased expression of neuronal markers beta-tubulin III, microtubule-associated protein-2, and growth-associated protein-43. PACAP38 induced stimulation of Rac and suppression of Rho GTPase activities. PACAP38 downregulated the nerve growth factor receptor (p75(NTR)) and associated Nogo-(Neurite outgrowth-A) receptor. Collectively, these in vitro and in vivo results propose that PACAP exhibits neuroprotective effects in cerebral ischemia by three mechanisms: a direct one, mediated by PACAP receptors, and two indirect, induced by neurotrophin release, activation of the trkB receptors and attenuation of neuronal growth inhibitory signaling molecules p75(NTR) and Nogo receptor.
Figures





Similar articles
-
PACAP38 differentially effects genes and CRMP2 protein expression in ischemic core and penumbra regions of permanent middle cerebral artery occlusion model mice brain.Int J Mol Sci. 2014 Sep 23;15(9):17014-34. doi: 10.3390/ijms150917014. Int J Mol Sci. 2014. PMID: 25257527 Free PMC article.
-
Molecular Mechanism for PACAP 38-Induced Neurite Outgrowth in PC12 Cells.Neural Plast. 2021 Aug 7;2021:2522454. doi: 10.1155/2021/2522454. eCollection 2021. Neural Plast. 2021. PMID: 34422037 Free PMC article.
-
Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat.Stroke. 2000 Jun;31(6):1411-7. doi: 10.1161/01.str.31.6.1411. Stroke. 2000. PMID: 10835464
-
Protective effects of PACAP in ischemia.J Headache Pain. 2018 Mar 2;19(1):19. doi: 10.1186/s10194-018-0845-3. J Headache Pain. 2018. PMID: 29500688 Free PMC article. Review.
-
Alternative Routes of Administration of the Neuroprotective Pituitary Adenylate Cyclase Activating Polypeptide.Curr Pharm Des. 2018;24(33):3892-3904. doi: 10.2174/1381612824666181112110934. Curr Pharm Des. 2018. PMID: 30417775 Review.
Cited by
-
Intranasal Administration of PACAP Is an Efficient Delivery Route to Reduce Infarct Volume and Promote Functional Recovery After Transient and Permanent Middle Cerebral Artery Occlusion.Front Endocrinol (Lausanne). 2021 Jan 20;11:585082. doi: 10.3389/fendo.2020.585082. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33551991 Free PMC article.
-
C-terminal amidation of PACAP-38 and PACAP-27 is dispensable for biological activity at the PAC1 receptor.Peptides. 2016 May;79:39-48. doi: 10.1016/j.peptides.2016.03.003. Epub 2016 Mar 11. Peptides. 2016. PMID: 26976270 Free PMC article.
-
A comprehensive p75 neurotrophin receptor gene network and pathway analyses identifying new target genes.Sci Rep. 2020 Sep 11;10(1):14984. doi: 10.1038/s41598-020-72061-z. Sci Rep. 2020. PMID: 32917932 Free PMC article.
-
PACAP protects against salsolinol-induced toxicity in dopaminergic SH-SY5Y cells: implication for Parkinson's disease.J Mol Neurosci. 2013 Jul;50(3):600-7. doi: 10.1007/s12031-013-0015-7. Epub 2013 Apr 28. J Mol Neurosci. 2013. PMID: 23625270 Free PMC article.
-
Astilbin exerts a neuroprotective effect by upregulating the signaling of nuclear NF-E2-related factor 2 in vitro.Heliyon. 2024 Sep 3;10(17):e37276. doi: 10.1016/j.heliyon.2024.e37276. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296123 Free PMC article.
References
-
- Arien-Zakay H, Lecht S, Bercu MM, et al. Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp Neurol. 2009;216:83–94. - PubMed
-
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. - PubMed
-
- Benowitz LI, Perrone-Bizzozero NI, Neve RL, Rodriguez W. GAP-43 as a marker for structural plasticity in the mature CNS. Prog Brain Res. 1990;86:309–320. - PubMed
-
- Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. - PubMed
-
- Botia B, Basille M, Allais A, et al. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007;28:1746–1752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

