Dopamine and full-field illumination activate D1 and D2-D5-type receptors in adult rat retinal ganglion cells
- PMID: 22678972
- PMCID: PMC3538137
- DOI: 10.1002/cne.23159
Dopamine and full-field illumination activate D1 and D2-D5-type receptors in adult rat retinal ganglion cells
Abstract
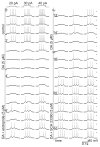
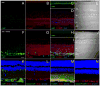
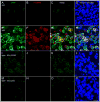
Dopamine can regulate signal generation and transmission by activating multiple receptors and signaling cascades, especially in striatum, hippocampus, and cerebral cortex. Dopamine modulates an even larger variety of cellular properties in retina, yet has been reported to do so by only D1 receptor-driven cyclic adenosine monophosphate (cAMP) increases or D2 receptor-driven cAMP decreases. Here, we test the possibility that dopamine operates differently on retinal ganglion cells, because the ganglion cell layer binds D1 and D2 receptor ligands, and displays changes in signaling components other than cAMP under illumination that should release dopamine. In adult rat retinal ganglion cells, based on patch-clamp recordings, Ca(2+) imaging, and immunohistochemistry, we find that 1) spike firing is inhibited by dopamine and SKF 83959 (an agonist that does not activate homomeric D1 receptors or alter cAMP levels in other systems); 2) D1 and D2 receptor antagonists (SCH 23390, eticlopride, raclopride) counteract these effects; 3) these antagonists also block light-induced rises in cAMP, light-induced activation of Ca(2+) /calmodulin-dependent protein kinase II, and dopamine-induced Ca(2+) influx; and 4) the Ca(2+) rise is markedly reduced by removing extracellular Ca(2+) and by an IP3 receptor antagonist (2-APB). These results provide the first evidence that dopamine activates a receptor in adult mammalian retinal neurons that is distinct from classical D1 and D2 receptors, and that dopamine can activate mechanisms in addition to cAMP and cAMP-dependent protein kinase to modulate retinal ganglion cell excitability.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
No identified conflicts.
Figures

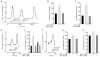
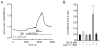
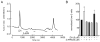

References
-
- Abdel-Majid RM, Tremblay F, Baldridge WH. Localization of adenylyl cyclase proteins in the rodent retina. Mol Brain Res. 2002;101:62–70. - PubMed
-
- Berrera M, Dodoni G, Monterisi S, Pertegato V, Zamparo I, Zaccolo M. A toolkit for real-time detection of cAMP: insights into compartmentalized signaling. Handb Exp Pharmacol. 2008;186:285–298. - PubMed
-
- Bjelke B, Goldstein M, Tinner B, Andersson C, Sesack SR, Steinbusch HW, Lew JY, He X, Watson S, Tengroth B, Fuxe K. Dopaminergic transmission in the rat retina: evidence for volume transmission. J Chem Neuroanat. 1996;12:37–50. - PubMed
-
- Brainard GC, Morgan WW. Light-induced stimulation of retinal dopamine: a dose-response relationship. Brain Res. 1987;424:199–203. - PubMed
-
- Brann MR, Young WS., III Dopamine receptors are located on rods in bovine retina. Neurosci Lett. 1986;69:221–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

