Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells
- PMID: 22679022
- PMCID: PMC3406659
- DOI: 10.1074/jbc.M112.361360
Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells
Abstract
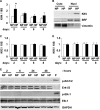
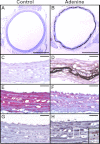
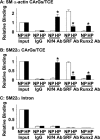
Hyperphosphatemia in chronic kidney disease is highly associated with vascular calcification. Previous studies have shown that high phosphate-induced phenotypic switching of vascular smooth muscle cells (SMCs) into osteogenic cells plays an important role in the calcification process. In the present study, we determined whether Krüppel-like factor 4 (Klf4) and phosphorylated Elk-1, transcriptional repressors of SMC differentiation marker genes activated by intimal atherogenic stimuli, contributed to this process. Rat aortic SMCs were cultured in the medium with normal (0.9 mmol/liter) or high (4.5 mmol/liter) phosphate concentration. Results showed that high phosphate concentration induced SMC calcification. Moreover, high phosphate decreased expression of SMC differentiation marker genes including smooth muscle α-actin and SM22α, whereas it increased expression of osteogenic genes, such as Runx2 and osteopontin. High phosphate also induced Klf4 expression, although it did not phosphorylate Elk-1. In response to high phosphate, Klf4 selectively bound to the promoter regions of SMC differentiation marker genes. Of importance, siRNA-mediated knockdown of Klf4 blunted high phosphate-induced suppression of SMC differentiation marker genes, as well as increases in expression of osteogenic genes and calcium deposition. Klf4 was also induced markedly in the calcified aorta of adenine-induced uremic rats. Results provide novel evidence that Klf4 mediates high phosphate-induced conversion of SMCs into osteogenic cells.
Figures



Similar articles
-
Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids.Am J Physiol Cell Physiol. 2008 Nov;295(5):C1175-82. doi: 10.1152/ajpcell.00288.2008. Epub 2008 Sep 3. Am J Physiol Cell Physiol. 2008. PMID: 18768922 Free PMC article.
-
Vascular calcification is coupled with phenotypic conversion of vascular smooth muscle cells through Klf5-mediated transactivation of the Runx2 promoter.Biosci Rep. 2014 Nov 4;34(6):e00148. doi: 10.1042/BSR20140103. Biosci Rep. 2014. PMID: 25205373 Free PMC article.
-
Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro.Arterioscler Thromb Vasc Biol. 2014 May;34(5):1002-10. doi: 10.1161/ATVBAHA.114.303301. Epub 2014 Feb 27. Arterioscler Thromb Vasc Biol. 2014. PMID: 24578384
-
Key regulators of vascular calcification in chronic kidney disease: Hyperphosphatemia, BMP2, and RUNX2.PeerJ. 2024 Sep 19;12:e18063. doi: 10.7717/peerj.18063. eCollection 2024. PeerJ. 2024. PMID: 39308809 Free PMC article. Review.
-
Transcriptional regulation of vascular smooth muscle cell proliferation, differentiation and senescence: Novel targets for therapy.Vascul Pharmacol. 2022 Oct;146:107091. doi: 10.1016/j.vph.2022.107091. Epub 2022 Jul 25. Vascul Pharmacol. 2022. PMID: 35896140 Review.
Cited by
-
Microcalcification and Thoracic Aortopathy: A Window Into Disease Severity.Arterioscler Thromb Vasc Biol. 2022 Aug;42(8):1048-1059. doi: 10.1161/ATVBAHA.122.317111. Epub 2022 Jun 30. Arterioscler Thromb Vasc Biol. 2022. PMID: 35770666 Free PMC article.
-
Inhibition of Notch signaling enhances transdifferentiation of the esophageal squamous epithelium towards a Barrett's-like metaplasia via KLF4.Cell Cycle. 2014;13(24):3857-66. doi: 10.4161/15384101.2014.972875. Cell Cycle. 2014. PMID: 25558829 Free PMC article.
-
KLF4 Knockdown Attenuates TBI-Induced Neuronal Damage through p53 and JAK-STAT3 Signaling.CNS Neurosci Ther. 2017 Feb;23(2):106-118. doi: 10.1111/cns.12633. Epub 2016 Sep 27. CNS Neurosci Ther. 2017. PMID: 27671232 Free PMC article.
-
Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic microRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia.Nutrients. 2022 Jun 22;14(13):2589. doi: 10.3390/nu14132589. Nutrients. 2022. PMID: 35807767 Free PMC article.
-
Biomarkers of mineral metabolism and progression of aortic valve and mitral annular calcification: The Multi-Ethnic Study of Atherosclerosis.Atherosclerosis. 2019 Jun;285:79-86. doi: 10.1016/j.atherosclerosis.2019.04.215. Epub 2019 Apr 13. Atherosclerosis. 2019. PMID: 31048102 Free PMC article.
References
-
- Tonelli M., Wiebe N., Culleton B., House A., Rabbat C., Fok M., McAlister F., Garg A. X. (2006) Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 17, 2034–2047 - PubMed
-
- London G. M., Guérin A. P., Marchais S. J., Métivier F., Pannier B., Adda H. (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 18, 1731–1740 - PubMed
-
- Moe S. M., O'Neill K. D., Duan D., Ahmed S., Chen N. X., Leapman S. B., Fineberg N., Kopecky K. (2002) Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 61, 638–647 - PubMed
-
- Shioi A., Nishizawa Y., Jono S., Koyama H., Hosoi M., Morii H. (1995) β-Glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 15, 2003–2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

