GSK-3 promotes cell survival, growth, and PAX3 levels in human melanoma cells
- PMID: 22679108
- PMCID: PMC3422428
- DOI: 10.1158/1541-7786.MCR-11-0387
GSK-3 promotes cell survival, growth, and PAX3 levels in human melanoma cells
Abstract
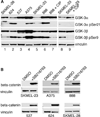
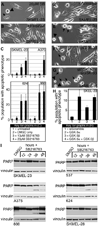
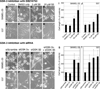
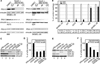
GSK-3 is a serine/threonine kinase involved in a diverse range of cellular processes. GSK-3 exists in two isoforms, GSK-3α and GSK-3β, which possess some functional redundancy but also play distinct roles depending on developmental and cellular context. In this article, we found that GSK-3 actively promoted cell growth and survival in melanoma cells, and blocking this activity with small-molecule inhibitor SB216763 or gene-specific siRNA decreased proliferation, increased apoptosis, and altered cellular morphology. These alterations coincided with loss of PAX3, a transcription factor implicated in proliferation, survival, and migration of developing melanoblasts. We further found that PAX3 directly interacted with and was phosphorylated in vitro on a number of residues by GSK-3β. In melanoma cells, direct inhibition of PAX3 lead to cellular changes that paralleled the response to GSK-3 inhibition. Maintenance of PAX3 expression protected melanoma cells from the anti-tumor effects of SB216763. These data support a model wherein GSK-3 regulates proliferation and morphology of melanoma through phosphorylation and increased levels of PAX3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Inhibition of GSK-3beta ameliorates hepatic ischemia-reperfusion injury through GSK-3beta/beta-catenin signaling pathway in mice.Hepatobiliary Pancreat Dis Int. 2012 Jun;11(3):278-84. doi: 10.1016/s1499-3872(12)60161-1. Hepatobiliary Pancreat Dis Int. 2012. PMID: 22672822
-
Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells.Mol Cancer Ther. 2007 Mar;6(3):1151-8. doi: 10.1158/1535-7163.MCT-06-0665. Mol Cancer Ther. 2007. PMID: 17363508
-
GSK-3 inhibitor inhibits cell proliferation and induces apoptosis in human osteosarcoma cells.Oncol Rep. 2016 Apr;35(4):2348-54. doi: 10.3892/or.2016.4565. Epub 2016 Jan 15. Oncol Rep. 2016. PMID: 26781995
-
PAX3 across the spectrum: from melanoblast to melanoma.Crit Rev Biochem Mol Biol. 2009 Jun;44(2-3):85-97. doi: 10.1080/10409230902755056. Crit Rev Biochem Mol Biol. 2009. PMID: 19401874 Review.
-
Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease.Pigment Cell Melanoma Res. 2008 Dec;21(6):627-45. doi: 10.1111/j.1755-148X.2008.00514.x. Pigment Cell Melanoma Res. 2008. PMID: 18983540 Free PMC article. Review.
Cited by
-
PAX3 and FOXD3 Promote CXCR4 Expression in Melanoma.J Biol Chem. 2015 Sep 4;290(36):21901-14. doi: 10.1074/jbc.M115.670976. Epub 2015 Jul 23. J Biol Chem. 2015. PMID: 26205821 Free PMC article.
-
Targeting Pan-ETS Factors Inhibits Melanoma Progression.Cancer Res. 2021 Apr 15;81(8):2071-2085. doi: 10.1158/0008-5472.CAN-19-1668. Epub 2021 Feb 1. Cancer Res. 2021. PMID: 33526511 Free PMC article.
-
Glycogen Synthase Kinase 3β in Cancer Biology and Treatment.Cells. 2020 Jun 3;9(6):1388. doi: 10.3390/cells9061388. Cells. 2020. PMID: 32503133 Free PMC article. Review.
-
Multiple alternative splicing and differential expression pattern of the glycogen synthase kinase-3β (GSK3β) gene in goat (Capra hircus).PLoS One. 2014 Oct 15;9(10):e109555. doi: 10.1371/journal.pone.0109555. eCollection 2014. PLoS One. 2014. PMID: 25334049 Free PMC article.
-
BioID-based intact cell interactome of the Kv1.3 potassium channel identifies a Kv1.3-STAT3-p53 cellular signaling pathway.Sci Adv. 2024 Sep 6;10(36):eadn9361. doi: 10.1126/sciadv.adn9361. Epub 2024 Sep 4. Sci Adv. 2024. PMID: 39231216 Free PMC article.
References
-
- Yao HB, Shaw PC, Wong CC, Wan DC. Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. J Chem Neuroanat. 2002;23:291–297. - PubMed
-
- Lau KF, Miller CC, Anderton BH, Shaw PC. Expression analysis of glycogen synthase kinase-3 in human tissues. J Pept Res. 1999;54:85–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

