In vivo models of primary brain tumors: pitfalls and perspectives
- PMID: 22679124
- PMCID: PMC3408261
- DOI: 10.1093/neuonc/nos135
In vivo models of primary brain tumors: pitfalls and perspectives
Abstract
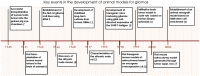
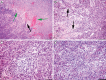
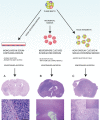
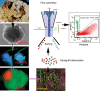
Animal modeling for primary brain tumors has undergone constant development over the last 60 years, and significant improvements have been made recently with the establishment of highly invasive glioblastoma models. In this review we discuss the advantages and pitfalls of model development, focusing on chemically induced models, various xenogeneic grafts of human cell lines, including stem cell-like cell lines and biopsy spheroids. We then discuss the development of numerous genetically engineered models available to study mechanisms of tumor initiation and progression. At present it is clear that none of the current animal models fully reflects human gliomas. Yet, the various model systems have provided important insight into specific mechanisms of tumor development. In particular, it is anticipated that a combined comprehensive knowledge of the various models currently available will provide important new knowledge on target identification and the validation and development of new therapeutic strategies.
Figures
References
-
- Kerbel RS. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 1998;17(3):301–304. - PubMed
-
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived—but they can be improved. Cancer Biol Ther. 2003;2(4 suppl 1):S134–S139. - PubMed
-
- Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40(6):837–844. - PubMed
-
- Hesselager G, Holland EC. Using mice to decipher the molecular genetics of brain tumors. Neurosurgery. 2003;53(3):685–694. discussion 695. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

