Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias
- PMID: 22679140
- PMCID: PMC3789535
- DOI: 10.1161/CIRCRESAHA.111.243956
Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias
Abstract
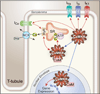
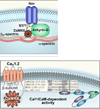
Understanding relationships between heart failure and arrhythmias, important causes of suffering and sudden death, remains an unmet goal for biomedical researchers and physicians. Evidence assembled over the past decade supports a view that activation of the multifunctional Ca(2+) and calmodulin-dependent protein kinase II (CaMKII) favors myocardial dysfunction and cell membrane electrical instability. CaMKII activation follows increases in intracellular Ca(2+) or oxidation, upstream signals with the capacity to transition CaMKII into a Ca(2+) and calmodulin-independent constitutively active enzyme. Constitutively active CaMKII appears poised to participate in disease pathways by catalyzing the phosphorylation of classes of protein targets important for excitation-contraction coupling and cell survival, including ion channels and Ca(2+) homeostatic proteins, and transcription factors that drive hypertrophic and inflammatory gene expression. This rich diversity of downstream targets helps to explain the potential for CaMKII to simultaneously affect mechanical and electrical properties of heart muscle cells. Proof-of-concept studies from a growing number of investigators show that CaMKII inhibition is beneficial for improving myocardial performance and for reducing arrhythmias. We review the molecular physiology of CaMKII and discuss CaMKII actions at key cellular targets and results of animal models of myocardial hypertrophy, dysfunction, and arrhythmias that suggest CaMKII inhibition may benefit myocardial function while reducing arrhythmias.
Figures



References
-
- Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Clinical Cardiology, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on High Blood Pressure Research, Quality of Care Outcomes Research Interdisciplinary Working Group, Functional Genomics and Translational Biology Interdisciplinary Working Group. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research. Circulation. 2008;117(19):2544–2565. - PubMed
-
- Members WG, Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J Executive Summary: Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Committee OBOTAHAS Subcommittee SS Roger VL Turner MB Disease OBOTAHAH Group SSW. Circulation. 2011;123(4):459–463. - PMC - PubMed
-
- Lloyd-Jones D, Adams R, Brown T, Carnethon M. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 - PubMed
-
- Krell MJ, Kline EM, Bates ER, Hodgson JM, Dilworth LR, Laufer N, Vogel RA, Pitt B. Intermittent, ambulatory dobutamine infusions in patients with severe congestive heart failure. Am. Heart J. 1986;112(4):787–791. - PubMed
-
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324(12):781–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

