Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn
- PMID: 22679221
- PMCID: PMC3400831
- DOI: 10.1124/pr.111.004770
Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn
Abstract
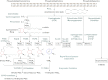
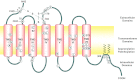
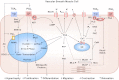
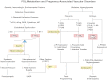
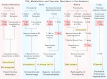
Prostacyclin (PGI(2)) is a member of the prostanoid group of eicosanoids that regulate homeostasis, hemostasis, smooth muscle function and inflammation. Prostanoids are derived from arachidonic acid by the sequential actions of phospholipase A(2), cyclooxygenase (COX), and specific prostaglandin (PG) synthases. There are two major COX enzymes, COX1 and COX2, that differ in structure, tissue distribution, subcellular localization, and function. COX1 is largely constitutively expressed, whereas COX2 is induced at sites of inflammation and vascular injury. PGI(2) is produced by endothelial cells and influences many cardiovascular processes. PGI(2) acts mainly on the prostacyclin (IP) receptor, but because of receptor homology, PGI(2) analogs such as iloprost may act on other prostanoid receptors with variable affinities. PGI(2)/IP interaction stimulates G protein-coupled increase in cAMP and protein kinase A, resulting in decreased [Ca(2+)](i), and could also cause inhibition of Rho kinase, leading to vascular smooth muscle relaxation. In addition, PGI(2) intracrine signaling may target nuclear peroxisome proliferator-activated receptors and regulate gene transcription. PGI(2) counteracts the vasoconstrictor and platelet aggregation effects of thromboxane A(2) (TXA(2)), and both prostanoids create an important balance in cardiovascular homeostasis. The PGI(2)/TXA(2) balance is particularly critical in the regulation of maternal and fetal vascular function during pregnancy and in the newborn. A decrease in PGI(2)/TXA(2) ratio in the maternal, fetal, and neonatal circulation may contribute to preeclampsia, intrauterine growth restriction, and persistent pulmonary hypertension of the newborn (PPHN), respectively. On the other hand, increased PGI(2) activity may contribute to patent ductus arteriosus (PDA) and intraventricular hemorrhage in premature newborns. These observations have raised interest in the use of COX inhibitors and PGI(2) analogs in the management of pregnancy-associated and neonatal vascular disorders. The use of aspirin to decrease TXA(2) synthesis has shown little benefit in preeclampsia, whereas indomethacin and ibuprofen are used effectively to close PDA in the premature newborn. PGI(2) analogs have been used effectively in primary pulmonary hypertension in adults and have shown promise in PPHN. Careful examination of PGI(2) metabolism and the complex interplay with other prostanoids will help design specific modulators of the PGI(2)-dependent pathways for the management of pregnancy-related and neonatal vascular disorders.
Figures



References
-
- Abman SH, Stenmark KR. (1992) Changes in lung eicosanoid content during normal and abnormal transition in perinatal lambs. Am J Physiol 262:L214–L222 - PubMed
-
- Afshari A, Brok J, Møller AM, Wetterslev J. (2010) Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev (8):CD007733. - PubMed
-
- Alano MA, Ngougmna E, Ostrea EM, Jr, Konduri GG. (2001) Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics 107:519–523 - PubMed
-
- Ali FY, Egan K, FitzGerald GA, Desvergne B, Wahli W, Bishop-Bailey D, Warner TD, Mitchell JA. (2006) Role of prostacyclin versus peroxisome proliferator-activated receptor beta receptors in prostacyclin sensing by lung fibroblasts. Am J Respir Cell Mol Biol 34:242–246 - PubMed
-
- Arehart E, Gleim S, Kasza Z, Fetalvero KM, Martin KA, Hwa J. (2007) Prostacyclin, atherothrombosis, and cardiovascular disease. Curr Med Chem 14:2161–2169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

