Neonatal diethylstilbestrol exposure alters the metabolic profile of uterine epithelial cells
- PMID: 22679223
- PMCID: PMC3484869
- DOI: 10.1242/dmm.009076
Neonatal diethylstilbestrol exposure alters the metabolic profile of uterine epithelial cells
Abstract
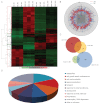
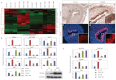
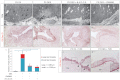
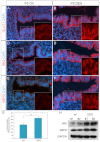
Developmental exposure to diethylstilbestrol (DES) causes reproductive tract malformations, affects fertility and increases the risk of clear cell carcinoma of the vagina and cervix in humans. Previous studies on a well-established mouse DES model demonstrated that it recapitulates many features of the human syndrome, yet the underlying molecular mechanism is far from clear. Using the neonatal DES mouse model, the present study uses global transcript profiling to systematically explore early gene expression changes in individual epithelial and mesenchymal compartments of the neonatal uterus. Over 900 genes show differential expression upon DES treatment in either one or both tissue layers. Interestingly, multiple components of peroxisome proliferator-activated receptor-γ (PPARγ)-mediated adipogenesis and lipid metabolism, including PPARγ itself, are targets of DES in the neonatal uterus. Transmission electron microscopy and Oil-Red O staining further demonstrate a dramatic increase in lipid deposition in uterine epithelial cells upon DES exposure. Neonatal DES exposure also perturbs glucose homeostasis in the uterine epithelium. Some of these neonatal DES-induced metabolic changes appear to last into adulthood, suggesting a permanent effect of DES on energy metabolism in uterine epithelial cells. This study extends the list of biological processes that can be regulated by estrogen or DES, and provides a novel perspective for endocrine disruptor-induced reproductive abnormalities.
Figures
Similar articles
-
Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis.Development. 2004 Apr;131(7):1639-49. doi: 10.1242/dev.01038. Epub 2004 Mar 3. Development. 2004. PMID: 14998922
-
Morphological effects of diethylstilbestrol on neonatal mouse uterus and vagina.Cancer Res. 1981 Nov;41(11 Pt 1):4667-77. Cancer Res. 1981. PMID: 7306983
-
Response of xenografts of developing human female reproductive tracts to the synthetic estrogen, diethylstilbestrol.Differentiation. 2017 Nov-Dec;98:35-54. doi: 10.1016/j.diff.2017.10.001. Epub 2017 Oct 4. Differentiation. 2017. PMID: 29102757 Free PMC article.
-
Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract.Toxicology. 2004 Dec 1;205(1-2):55-63. doi: 10.1016/j.tox.2004.06.046. Toxicology. 2004. PMID: 15458790 Review.
-
Expression of estrogen-regulated genes during development in the mouse uterus exposed to diethylstilbestrol neonatally.Curr Pharm Des. 2006;12(12):1505-20. doi: 10.2174/138161206776389840. Curr Pharm Des. 2006. PMID: 16611131 Review.
Cited by
-
(Endo)Cannabinoids and Gynaecological Cancers.Cancers (Basel). 2020 Dec 25;13(1):37. doi: 10.3390/cancers13010037. Cancers (Basel). 2020. PMID: 33375539 Free PMC article. Review.
-
EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals.Endocr Rev. 2015 Dec;36(6):E1-E150. doi: 10.1210/er.2015-1010. Epub 2015 Nov 6. Endocr Rev. 2015. PMID: 26544531 Free PMC article. Review.
-
Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment.Endocr Rev. 2022 Jul 13;43(4):678-719. doi: 10.1210/endrev/bnab039. Endocr Rev. 2022. PMID: 34741454 Free PMC article. Review.
-
Altered gene expression patterns during the initiation and promotion stages of neonatally diethylstilbestrol-induced hyperplasia/dysplasia/neoplasia in the hamster uterus.Reprod Toxicol. 2014 Dec;50:68-86. doi: 10.1016/j.reprotox.2014.09.002. Epub 2014 Sep 19. Reprod Toxicol. 2014. PMID: 25242112 Free PMC article.
-
Homeodomain Transcription Factor Msx-2 Regulates Uterine Progenitor Cell Response to Diethylstilbestrol.J Stem Cell Transplant Biol. 2015;1(1):105. doi: 10.19104/jstb.2015.105. Epub 2015 May 12. J Stem Cell Transplant Biol. 2015. PMID: 26457333 Free PMC article.
References
-
- Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. (1999). PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 - PubMed
-
- Bigsby R. M., Cooke P. S., Cunha G. R. (1986). A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am. J. Physiol. 251, E630–E636 - PubMed
-
- Bourke J. E., Dank S., Wilce P. A., Martin L. (1991). Effect of estradiol and progesterone on phosphatidylinositol metabolism in the uterine epithelium of the mouse. J. Steroid Biochem. Mol. Biol. 39, 337–342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

