Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology
- PMID: 22679224
- PMCID: PMC3484865
- DOI: 10.1242/dmm.008557
Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology
Abstract
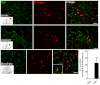
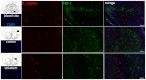
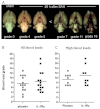
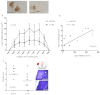
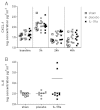
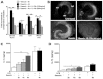
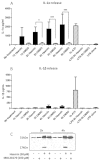
Subarachnoid haemorrhage (SAH) is a major contributor to the burden of stroke on society. Treatment options are limited and animal models of SAH do not always mimic key pathophysiological hallmarks of the disease, thus hindering development of new therapeutics. Inflammation is strongly associated with brain injury after SAH in animals and patients, and inhibition of the pro-inflammatory cytokine interleukin-1 (IL-1) represents a possible therapeutic target. Here we report that a rupture of the middle cerebral artery in the rat produces heterogeneous infarct patterns similar to those observed in human SAH. Administration of the IL-1 receptor antagonist (IL-1Ra) reduced blood-brain barrier breakdown, and the extent of breakdown correlated with brain injury. After SAH, haem oxygenase-1 (HO-1) was strongly expressed around the bleed site and in the cortex and striatum, indicating the presence of free haem, a breakdown product of haemoglobin. HO-1 expression was also found in the same regions as microglial/macrophage expression of IL-1α. The direct effect of haem on IL-1α expression was confirmed in vitro using organotypic slice culture (OSC). Haem-induced cell death was dependent on IL-1 signalling, with IL-1Ra completely blocking cellular injury. Furthermore, stimulation of mouse primary mixed glial cells with haem induced the release of IL-1α, but not IL-1β. Thus, we suggest that haem, released from lysed red blood cells (RBCs) in the subarachnoid space, acts as a danger-associated molecular pattern (DAMP) driving IL-1-dependent inflammation. These data provide new insights into inflammation after SAH-induced brain injury and suggest IL-1Ra as a candidate therapeutic for the disease.
Figures
References
-
- Allan S. M., Tyrrell P. J., Rothwell N. J. (2005). Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 5, 629–640 - PubMed
-
- Ascenzi P., Bocedi A., Visca P., Altruda F., Tolosano E., Beringhelli T., Fasano M. (2005). Hemoglobin and heme scavenging. IUBMB Life 57, 749–759 - PubMed
-
- Borsody M., Burke A., Coplin W., Miller-Lotan R., Levy A. (2006). Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology 66, 634–640 - PubMed
-
- Brough D., Tyrrell P. J., Allan S. M. (2011). Regulation of interleukin-1 in acute brain injury. Trends Pharmacol. Sci. 32, 617–622 - PubMed
-
- Chaichana K. L., Pradilla G., Huang J., Tamargo R. J. (2010). Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 73, 22–41 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

