Portable microfluidic chip for detection of Escherichia coli in produce and blood
- PMID: 22679370
- PMCID: PMC3368510
- DOI: 10.2147/IJN.S29629
Portable microfluidic chip for detection of Escherichia coli in produce and blood
Abstract
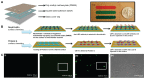
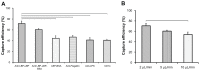
Pathogenic agents can lead to severe clinical outcomes such as food poisoning, infection of open wounds, particularly in burn injuries and sepsis. Rapid detection of these pathogens can monitor these infections in a timely manner improving clinical outcomes. Conventional bacterial detection methods, such as agar plate culture or polymerase chain reaction, are time-consuming and dependent on complex and expensive instruments, which are not suitable for point-of-care (POC) settings. Therefore, there is an unmet need to develop a simple, rapid method for detection of pathogens such as Escherichia coli. Here, we present an immunobased microchip technology that can rapidly detect and quantify bacterial presence in various sources including physiologically relevant buffer solution (phosphate buffered saline [PBS]), blood, milk, and spinach. The microchip showed reliable capture of E. coli in PBS with an efficiency of 71.8% ± 5% at concentrations ranging from 50 to 4,000 CFUs/mL via lipopolysaccharide binding protein. The limits of detection of the microchip for PBS, blood, milk, and spinach samples were 50, 50, 50, and 500 CFUs/mL, respectively. The presented technology can be broadly applied to other pathogens at the POC, enabling various applications including surveillance of food supply and monitoring of bacteriology in patients with burn wounds.
Keywords: Escherichia coli; food safety; microchip; point-of-care; sepsis.
Figures



Similar articles
-
Portable microfluidic integrated plasmonic platform for pathogen detection.Sci Rep. 2015 Mar 24;5:9152. doi: 10.1038/srep09152. Sci Rep. 2015. PMID: 25801042 Free PMC article.
-
Microfluidic Chip for Detection of Fungal Infections.ACS Omega. 2019 Apr 30;4(4):7474-7481. doi: 10.1021/acsomega.9b00499. Epub 2019 Apr 24. ACS Omega. 2019. PMID: 31080939 Free PMC article.
-
Simple quantitative analysis of Escherichia coli K-12 internalized in baby spinach using Fourier Transform Infrared spectroscopy.Int J Food Microbiol. 2010 Nov 15;144(1):147-51. doi: 10.1016/j.ijfoodmicro.2010.09.013. Int J Food Microbiol. 2010. PMID: 20937537
-
Methods for the detection and isolation of Shiga toxin-producing Escherichia coli.Symp Ser Soc Appl Microbiol. 2000;(29):133S-143S. doi: 10.1111/j.1365-2672.2000.tb05341.x. Symp Ser Soc Appl Microbiol. 2000. PMID: 10880188 Review.
-
Review of the detection of pathogenic Escherichia coli based-microchip technology.Anal Sci. 2025 Mar;41(3):225-236. doi: 10.1007/s44211-024-00693-6. Epub 2024 Dec 9. Anal Sci. 2025. PMID: 39654011 Review.
Cited by
-
Diagnosis of Bloodstream Infections: An Evolution of Technologies towards Accurate and Rapid Identification and Antibiotic Susceptibility Testing.Antibiotics (Basel). 2022 Apr 12;11(4):511. doi: 10.3390/antibiotics11040511. Antibiotics (Basel). 2022. PMID: 35453262 Free PMC article. Review.
-
Construction of P-glycoprotein incorporated tethered lipid bilayer membranes.Biochem Biophys Rep. 2015 Jun 4;2:115-122. doi: 10.1016/j.bbrep.2015.05.012. eCollection 2015 Jul. Biochem Biophys Rep. 2015. PMID: 29124152 Free PMC article.
-
Recent Progress and Challenges on the Microfluidic Assay of Pathogenic Bacteria Using Biosensor Technology.Biomimetics (Basel). 2022 Oct 25;7(4):175. doi: 10.3390/biomimetics7040175. Biomimetics (Basel). 2022. PMID: 36412703 Free PMC article. Review.
-
Electroosmotic flow driven microfluidic device for bacteria isolation using magnetic microbeads.Sci Rep. 2019 Oct 2;9(1):14228. doi: 10.1038/s41598-019-50713-z. Sci Rep. 2019. PMID: 31578397 Free PMC article.
-
Nanostructured optical photonic crystal biosensor for HIV viral load measurement.Sci Rep. 2014 Feb 28;4:4116. doi: 10.1038/srep04116. Sci Rep. 2014. PMID: 24576941 Free PMC article.
References
-
- Schlichting D, McCollam JS. Recognizing and managing severe sepsis: a common and deadly threat. South Med J. 2007;100(6):594–600. - PubMed
-
- Crutchfield S, Roberts T. FoodReview: The 1990’s: a dynamic decade for the U.S. food system. Food Review Archives. 2000;23(3)
-
- Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365(19):1771–1780. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

