The origin of glucocorticoid hormone oscillations
- PMID: 22679394
- PMCID: PMC3367982
- DOI: 10.1371/journal.pbio.1001341
The origin of glucocorticoid hormone oscillations
Abstract
Oscillating levels of adrenal glucocorticoid hormones are essential for optimal gene expression, and for maintaining physiological and behavioural responsiveness to stress. The biological basis for these oscillations is not known, but a neuronal "pulse generator" within the hypothalamus has remained a popular hypothesis. We demonstrate that pulsatile hypothalamic activity is not required for generating ultradian glucocorticoid oscillations. We show that a constant level of corticotrophin-releasing hormone (CRH) can activate a dynamic pituitary-adrenal peripheral network to produce ultradian adrenocorticotrophic hormone and glucocorticoid oscillations with a physiological frequency. This oscillatory response to CRH is dose dependent and becomes disrupted for higher levels of CRH. These data suggest that glucocorticoid oscillations result from a sub-hypothalamic pituitary-adrenal system, which functions as a deterministic peripheral hormone oscillator with a characteristic ultradian frequency. This constitutes a novel mechanism by which the level, rather than the pattern, of CRH determines the dynamics of glucocorticoid hormone secretion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
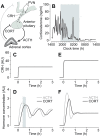
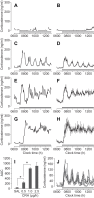
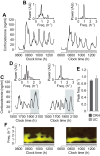
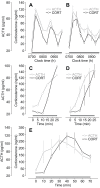
References
-
- Chrousos G. P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. - PubMed
-
- McEwen B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. - PubMed
-
- de Kloet E. R, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. - PubMed
-
- Sapolsky R. M, Romero L. M, Munck A. U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

