Differential response of immunohistochemically defined breast cancer subtypes to anthracycline-based adjuvant chemotherapy with or without paclitaxel
- PMID: 22679488
- PMCID: PMC3367950
- DOI: 10.1371/journal.pone.0037946
Differential response of immunohistochemically defined breast cancer subtypes to anthracycline-based adjuvant chemotherapy with or without paclitaxel
Abstract
Background: The aim of the present study was to investigate the efficacy of adjuvant dose-dense sequential chemotherapy with epirubicin, paclitaxel, and CMF in subgroups of patients with high-risk operable breast cancer, according to tumor subtypes defined by immunohistochemistry (IHC).
Materials and methods: Formalin-fixed paraffin-embedded (FFPE) tumor tissue samples from 1,039 patients participating in two adjuvant dose-dense sequential chemotherapy phase III trials were centrally assessed in tissue micro-arrays by IHC for 6 biological markers, that is, estrogen receptor (ER), progesterone receptor (PgR), HER2, Ki67, cytokeratin 5 (CK5), and EGFR. The majority of the cases were further evaluated for HER2 amplification by FISH. Patients were classified as: luminal A (ER/PgR-positive, HER2-negative, Ki67(low)); luminal B (ER/PgR-positive, HER2-negative, Ki67(high)); luminal-HER2 (ER/PgR-positive, HER2-positive); HER2-enriched (ER-negative, PgR-negative, HER2-positive); triple-negative (TNBC) (ER-negative, PgR-negative, HER2-negative); and basal core phenotype (BCP) (TNBC, CK5-positive and/or EGFR-positive).
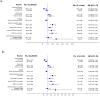
Results: After a median follow-up time of 105.4 months the 5-year disease-free survival (DFS) and overall survival (OS) rates were 73.1% and 86.1%, respectively. Among patients with HER2-enriched tumors there was a significant benefit in both DFS and OS (log-rank test; p = 0.021 and p = 0.006, respectively) for those treated with paclitaxel. The subtype classification was found to be of both predictive and prognostic value. Setting luminal A as the referent category, the adjusted for prognostic factors HR for relapse for patients with TNBC was 1.91 (95% CI: 1.31-2.80, Wald's p = 0.001) and for death 2.53 (95% CI: 1.62-3.60, p<0.001). Site of and time to first relapse differed according to subtype. Locoregional relapses and brain metastases were more frequent in patients with TNBC, while liver metastases were more often seen in patients with HER2-enriched tumors.
Conclusions: Triple-negative phenotype is of adverse prognostic value for DFS and OS in patients treated with adjuvant dose-dense sequential chemotherapy. In the pre-trastuzumab era, the HER2-enriched subtype predicts favorable outcome following paclitaxel-containing treatment.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

