Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization
- PMID: 22679502
- PMCID: PMC3367967
- DOI: 10.1371/journal.pone.0038408
Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization
Abstract
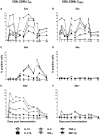
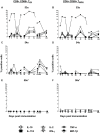
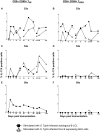
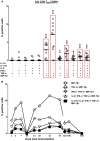
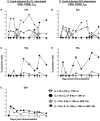
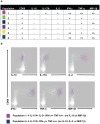
Salmonella enterica serovar Typhi (S. Typhi), the causative agent of typhoid fever, continues to cause significant morbidity and mortality world-wide. CD8+ T cells are an important component of the cell mediated immune (CMI) response against S. Typhi. Recently, interleukin (IL)-17A has been shown to contribute to mucosal immunity and protection against intracellular pathogens. To investigate multifunctional IL-17A responses against S. Typhi antigens in T memory subsets, we developed multiparametric flow cytometry methods to detect up to 6 cytokines/chemokines (IL-10, IL-17A, IL-2, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and macrophage inflammatory protein-1β (MIP-1β)) simultaneously. Five volunteers were immunized with a 4 dose regimen of live-attenuated S. Typhi vaccine (Ty21a), peripheral blood mononuclear cells (PBMC) were isolated before and at 11 time points after immunization, and CMI responses were evaluated. Of the 5 immunized volunteers studied, 3 produced detectable CD8+ T cell responses following stimulation with S. Typhi-infected autologous B lymphoblastoid cell lines (B-LCL). Additionally, 2 volunteers had detectable levels of intracellular cytokines in response to stimulation with S. Typhi-infected HLA-E restricted cells. Although the kinetics of the responses differed among volunteers, all of the responses were bi- or tri-phasic and included multifunctional CD8+ T cells. Virtually all of the IL-17A detected was derived from multifunctional CD8+ T cells. The presence of these multifunctional IL-17A+ CD8+ T cells was confirmed using an unsupervised analysis program, flow cytometry clustering without K (FLOCK). This is the first report of IL-17A production in response to S. Typhi in humans, indicating the presence of a Tc17 response which may be important in protection. The presence of IL-17A in multifunctional cells co-producing Tc1 cytokines (IL-2, IFN-γ and TNF-α) may also indicate that the distinction between Tc17 and Tc1 responses in humans is not as clearly delineated as suggested by in vitro experiments and animal models.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

