Altered circulating levels of matrix metalloproteinases and inhibitors associated with elevated type 2 cytokines in lymphatic filarial disease
- PMID: 22679524
- PMCID: PMC3367978
- DOI: 10.1371/journal.pntd.0001681
Altered circulating levels of matrix metalloproteinases and inhibitors associated with elevated type 2 cytokines in lymphatic filarial disease
Abstract
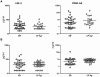
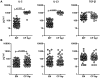
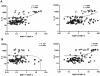
Background: Infection with Wuchereria bancrofti can cause severe disease characterized by subcutaneous fibrosis and extracellular matrix remodeling. Matrix metalloproteinases (MMPs) are a family of enzymes governing extracellular remodeling by regulating cellular homeostasis, inflammation, and tissue reorganization, while tissue-inhibitors of metalloproteinases (TIMPs) are endogenous regulators of MMPs. Homeostatic as well as inflammation-induced balance between MMPs and TIMPs is considered critical in mediating tissue pathology.
Methods: To elucidate the role of MMPs and TIMPs in filarial pathology, we compared the plasma levels of a panel of MMPs, TIMPs, other pro-fibrotic factors, and cytokines in individuals with chronic filarial pathology with (CP Ag+) or without (CP Ag-) active infection to those with clinically asymptomatic infections (INF) and in those without infection (endemic normal [EN]). Markers of pathogenesis were delineated based on comparisons between the two actively infected groups (CP Ag+ compared to INF) and those without active infection (CP Ag- compared to EN).
Results and conclusion: Our data reveal that an increase in circulating levels of MMPs and TIMPs is characteristic of the filarial disease process per se and not of active infection; however, filarial disease with active infection is specifically associated with increased ratios of MMP1/TIMP4 and MMP8/TIMP4 as well as with pro-fibrotic cytokines (IL-5, IL-13 and TGF-β). Our data therefore suggest that while filarial lymphatic disease is characterized by a non-specific increase in plasma MMPs and TIMPs, the balance between MMPs and TIMPs is an important factor in regulating tissue pathology during active infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Helminth Coinfection Is Associated With Enhanced Plasma Levels of Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases in Tuberculous Lymphadenitis.Front Cell Infect Microbiol. 2021 Jul 19;11:680665. doi: 10.3389/fcimb.2021.680665. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34350132 Free PMC article.
-
Comparison of localized versus systemic levels of Matrix metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and cytokines in tuberculous and non-tuberculous pleuritis patients.Hum Immunol. 2012 Oct;73(10):985-91. doi: 10.1016/j.humimm.2012.07.042. Epub 2012 Jul 20. Hum Immunol. 2012. PMID: 22820625 Free PMC article.
-
Circulating microbial products and acute phase proteins as markers of pathogenesis in lymphatic filarial disease.PLoS Pathog. 2012;8(6):e1002749. doi: 10.1371/journal.ppat.1002749. Epub 2012 Jun 7. PLoS Pathog. 2012. PMID: 22685406 Free PMC article.
-
An alternate perspective on the roles of TIMPs and MMPs in pathology.Am J Pathol. 2012 Jan;180(1):12-6. doi: 10.1016/j.ajpath.2011.09.008. Epub 2011 Oct 25. Am J Pathol. 2012. PMID: 22033229 Review.
-
The impact of matrix metalloproteinases and their tissue inhibitors in patients with chronic glaucoma - a literature review.Rom J Morphol Embryol. 2024 Oct-Dec;65(4):557-565. doi: 10.47162/RJME.65.4.01. Rom J Morphol Embryol. 2024. PMID: 39957016 Free PMC article. Review.
Cited by
-
Transcriptome-wide analysis of filarial extract-primed human monocytes reveal changes in LPS-induced PTX3 expression levels.Sci Rep. 2019 Feb 22;9(1):2562. doi: 10.1038/s41598-019-38985-x. Sci Rep. 2019. PMID: 30796272 Free PMC article.
-
Coincident helminth infection modulates systemic inflammation and immune activation in active pulmonary tuberculosis.PLoS Negl Trop Dis. 2014 Nov 6;8(11):e3289. doi: 10.1371/journal.pntd.0003289. eCollection 2014. PLoS Negl Trop Dis. 2014. PMID: 25375117 Free PMC article.
-
Helminth Coinfection Is Associated With Enhanced Plasma Levels of Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases in Tuberculous Lymphadenitis.Front Cell Infect Microbiol. 2021 Jul 19;11:680665. doi: 10.3389/fcimb.2021.680665. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34350132 Free PMC article.
-
Future Research Priorities for Morbidity Control of Lymphedema.Indian J Dermatol. 2017 Jan-Feb;62(1):33-40. doi: 10.4103/0019-5154.198039. Indian J Dermatol. 2017. PMID: 28216723 Free PMC article.
-
Eosinophils in filarial infections: Inducers of protection or pathology?Front Immunol. 2022 Oct 31;13:983812. doi: 10.3389/fimmu.2022.983812. eCollection 2022. Front Immunol. 2022. PMID: 36389745 Free PMC article. Review.
References
-
- Nutman TB, Kumaraswami V. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 2001;23:389–399. - PubMed
-
- Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000;16:544–548. - PubMed
-
- Figueredo-Silva J, Noroes J, Cedenho A, Dreyer G. The histopathology of bancroftian filariasis revisited: the role of the adult worm in the lymphatic-vessel disease. Ann Trop Med Parasitol. 2002;96:531–541. - PubMed
-
- Taylor MJ. Wolbachia in the inflammatory pathogenesis of human filariasis. Ann N Y Acad Sci. 2003;990:444–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

