Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects
- PMID: 22680341
- PMCID: PMC3394127
- DOI: 10.1111/j.1365-2125.2012.04164.x
Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects
Abstract
What is already known about this subject: • Cannabis based medicines are registered as a treatment for various indications, such as pain and spasms in multiple sclerosis (MS) patients, and anorexia and nausea in patients with HIV or receiving cancer treatment. • the pharmacokinetics of the various administration routes of cannabis and cannabis based medicines are variable and dosing is hard to regulate.
What this study adds: • Namisol is a new tablet containing pure THC (>98%) that has a beneficial pharmacokinetic profile after oral administration. • Namisol gives a quick onset of pharmacodynamic effects in healthy volunteers, which implies a rapid initiation of therapeutic effects in patients.
Aims: Among the main disadvantages of currently available Δ(9) -tetrahydrocannabinol (THC) formulations are dosing difficulties due to poor pharmacokinetic characteristics. Namisol® is a novel THC formulation, designed to improve THC absorption. The study objectives were to investigate the optimal administration route, pharmacokinetics (PK), pharmacodynamics (PD) and tolerability of Namisol®.
Methods: This first in human study consisted of two parts. Panel I included healthy males and females (n = 6/6) in a double-blind, double-dummy, randomized, crossover study with sublingual (crushed tablet) and oral administration of Namisol® (5 mg THC). Based on these results, male and female (n = 4/5) participants from panel I received oral THC 6.5 and 8.0 mg or matching placebo in a randomized, crossover, rising dose study during panel II. PD measurements were body sway; visual analogue scales (VAS) mood, psychedelic and heart rate. THC and 11-OH-THC population PK analysis was performed.
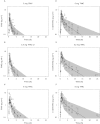
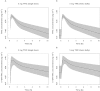
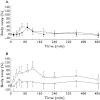
Results: Sublingual administration showed a flat concentration profile compared with oral administration. Oral THC apparent t(1/2) was 72-80 min, t(max) was 39-56 min and C(max) 2.92-4.69 ng ml(-1) . THC affected body sway (60.8%, 95% CI 29.5, 99.8), external perception (0.078 log mm, 95% CI 0.019, 0.137), alertness (-2.7 mm, 95% CI -4.5, -0.9) feeling high (0.256 log mm, 95% CI 0.093, 0.418) and heart rate (5.6 beats min(-1) , 95% CI 2.7, 6.5). Namisol® was well tolerated.
Conclusions: Oral Namisol® showed promising PK and PD characteristics. Variability and t(max) of THC plasma concentrations were smaller for Namisol® than reported for studies using oral dronabinol and nabilone. This study was performed in a limited number of healthy volunteers. Therefore, future research on Namisol® should study clinical effects in patient populations.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures

References
-
- Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse. 1987;7:39–50. - PubMed
-
- Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, Thompson A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003;362:1517–26. - PubMed
-
- Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, Simon RM, Rosenberg SA. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann Intern Med. 1979;91:819–24. - PubMed
-
- Orr LE, McKernan JF, Bloome B. Antiemetic effect of tetrahydrocannabinol compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Arch Intern Med. 1980;140:1431–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

