GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle
- PMID: 22681646
- PMCID: PMC3416696
- DOI: 10.1186/1475-2840-11-63
GLUT4, GLUT1, and GLUT8 are the dominant GLUT transcripts expressed in the murine left ventricle
Abstract
Background: The heart derives energy from a wide variety of substrates including fatty acids, carbohydrates, ketones, and amino acids. The healthy heart generates up to 30% of its ATP from glucose. Under conditions of cardiac injury or stress, the heart relies even more heavily on glucose as a source of fuel. Glucose is transported into the heart by members of the family of facilitative glucose transporters (GLUTs). While research examining the transport of glucose into the heart has primarily focused on the roles of the classical glucose transporters GLUT1 and GLUT4, little is known about the functions of more newly identified GLUT isoforms in the myocardium.
Methods: In this study the presence and relative RNA message abundance of each of the known GLUT isoforms was determined in left ventricular tissue from two commonly used inbred laboratory mouse strains (C57BL/6J and FVB/NJ) by quantitative real time PCR. Relative message abundance was also determined in GLUT4 null mice and in murine models of dilated and hypertrophic cardiomyopathy.
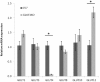
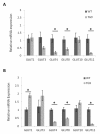
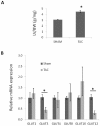
Results: GLUT4, GLUT1, and GLUT8 were found to be the most abundant GLUT transcripts in the normal heart, while GLUT3, GLUT10, and GLUT12 are present at relatively lower levels. Assessment of relative GLUT expression in left ventricular myocardium from mice with dilated cardiomyopathy revealed increased expression of GLUT1 with reduced levels of GLUT4, GLUT8, and GLUT12. Compensatory increase in the expression of GLUT12 was observed in genetically altered mice lacking GLUT4.
Conclusions: Glucose transporter expression varies significantly among murine models of cardiac dysfunction and involves several of the class III GLUT isoforms. Understanding how these more newly identified GLUT isoforms contribute to regulating myocardial glucose transport will enhance our comprehension of the normal physiology and pathophysiology of the heart.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

