Melanoma revives an embryonic migration program to promote plasticity and invasion
- PMID: 22681858
- PMCID: PMC3534779
- DOI: 10.1111/j.1755-148X.2012.01025.x
Melanoma revives an embryonic migration program to promote plasticity and invasion
Abstract
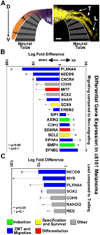
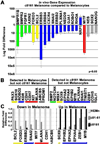
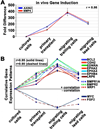
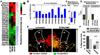
Cancer cells must regulate plasticity and invasion to survive and metastasize. However, the identification of targetable mechanisms to inhibit metastasis has been slow. Signaling programs that drive stem and progenitor cells during normal development offer an inroad to discover mechanisms common to metastasis. Using a chick embryo transplant model, we have compared molecular signaling programs of melanoma and their embryonic progenitors, the neural crest. We report that malignant melanoma cells hijack portions of the embryonic neural crest invasion program. Genes associated with neural crest induction, delamination, and migration are dynamically regulated by melanoma cells exposed to an embryonic neural crest microenvironment. Specifically, we demonstrate that metastatic melanoma cells exploit neural crest-related receptor tyrosine kinases to increase plasticity and facilitate invasion while primary melanocytes may actively suppress these responses under the same microenvironmental conditions. We conclude that aberrant regulation of neural crest developmental genes promotes plasticity and invasiveness in malignant melanoma.
© 2012 John Wiley & Sons A/S.
Figures



References
-
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical chemistry. 2009;55:611–522. - PubMed
-
- Duband JL, Monier F, Delannet M, Newgreen D. Epithelium-mesenchyme transition during neural crest development. Acta anatomica. 1995;154:63–78. - PubMed
-
- Gammill LS, Bronner-Fraser M. Genomic analysis of neural crest induction. Development (Cambridge, England) 2002;129:5731–5741. - PubMed
-
- Hamburger V, Hamilton HL. A Series of Normal Stages in the Development of the Chick Embryo. Journal of Morphology. 1951;88:49–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


