Triplex DNA-binding proteins are associated with clinical outcomes revealed by proteomic measurements in patients with colorectal cancer
- PMID: 22682314
- PMCID: PMC3537547
- DOI: 10.1186/1476-4598-11-38
Triplex DNA-binding proteins are associated with clinical outcomes revealed by proteomic measurements in patients with colorectal cancer
Abstract
Background: Tri- and tetra-nucleotide repeats in mammalian genomes can induce formation of alternative non-B DNA structures such as triplexes and guanine (G)-quadruplexes. These structures can induce mutagenesis, chromosomal translocations and genomic instability. We wanted to determine if proteins that bind triplex DNA structures are quantitatively or qualitatively different between colorectal tumor and adjacent normal tissue and if this binding activity correlates with patient clinical characteristics.
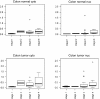
Methods: Extracts from 63 human colorectal tumor and adjacent normal tissues were examined by gel shifts (EMSA) for triplex DNA-binding proteins, which were correlated with clinicopathological tumor characteristics using the Mann-Whitney U, Spearman's rho, Kaplan-Meier and Mantel-Cox log-rank tests. Biotinylated triplex DNA and streptavidin agarose affinity binding were used to purify triplex-binding proteins in RKO cells. Western blotting and reverse-phase protein array were used to measure protein expression in tissue extracts.
Results: Increased triplex DNA-binding activity in tumor extracts correlated significantly with lymphatic disease, metastasis, and reduced overall survival. We identified three multifunctional splicing factors with biotinylated triplex DNA affinity: U2AF65 in cytoplasmic extracts, and PSF and p54nrb in nuclear extracts. Super-shift EMSA with anti-U2AF65 antibodies produced a shifted band of the major EMSA H3 complex, identifying U2AF65 as the protein present in the major EMSA band. U2AF65 expression correlated significantly with EMSA H3 values in all extracts and was higher in extracts from Stage III/IV vs. Stage I/II colon tumors (p=0.024). EMSA H3 values and U2AF65 expression also correlated significantly with GSK3 beta, beta-catenin, and NF- B p65 expression, whereas p54nrb and PSF expression correlated with c-Myc, cyclin D1, and CDK4. EMSA values and expression of all three splicing factors correlated with ErbB1, mTOR, PTEN, and Stat5. Western blots confirmed that full-length and truncated beta-catenin expression correlated with U2AF65 expression in tumor extracts.
Conclusions: Increased triplex DNA-binding activity in vitro correlates with lymph node disease, metastasis, and reduced overall survival in colorectal cancer, and increased U2AF65 expression is associated with total and truncated beta-catenin expression in high-stage colorectal tumors.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

