Pharmacodynamic analysis of tumour perfusion assessed by 15O-water-PET imaging during treatment with sunitinib malate in patients with advanced malignancies
- PMID: 22682364
- PMCID: PMC3468361
- DOI: 10.1186/2191-219X-2-31
Pharmacodynamic analysis of tumour perfusion assessed by 15O-water-PET imaging during treatment with sunitinib malate in patients with advanced malignancies
Abstract
Background: We evaluated pharmacodynamic changes in tumour perfusion using positron emission tomography (PET) imaging with 15O-water to assess biological response to sunitinib, a multitargeted tyrosine kinase inhibitor.
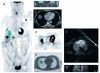
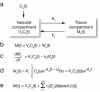
Methods: Patients with advanced malignancies received sunitinib 50 mg/day orally, once daily for 4 weeks on treatment, followed by 2 weeks off treatment, in repeated 6-week cycles. Quantitative measurement of tumour perfusion was assessed using 15O-water-PET at baseline and after 2 weeks of treatment. At least one reference tumour lesion was included in the fields of view and assessed at both time points. Patients also underwent 18 F-fluorodeoxyglucose (FDG)-PET imaging at baseline and after 2 and 4 weeks of treatment. Radiological response of the reference tumour lesion and overall radiological response were assessed at week 12. Serum pharmacokinetic and biomarker analyses were also performed.
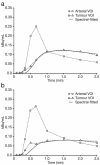
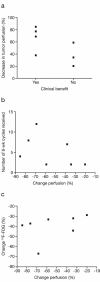
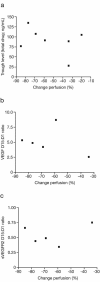
Results: Data were available for seven patients. Compared with baseline, all patients experienced a decrease in reference tumour blood flow ranging from 20 % to 85 % and also a reduction in the FDG standard uptake value ranging from 29 % to 67 %. Six patients experienced a partial metabolic response based on FDG-PET criteria. Four patients had stable disease defined by radiological response (Response Evaluation Criteria in Solid Tumors) lasting between 4 and 12 cycles. An association between perfusion change and clinical benefit, and biomarker levels including vascular endothelial growth factor was observed.
Conclusion: Administering sunitinib to patients with advanced malignancies is associated with early biological responses, including decreased blood flow in secondary tumour deposits.
Figures
References
LinkOut - more resources
Full Text Sources

