The effects of low dose X-irradiation on osteoblastic MC3T3-E1 cells in vitro
- PMID: 22682502
- PMCID: PMC3414775
- DOI: 10.1186/1471-2474-13-94
The effects of low dose X-irradiation on osteoblastic MC3T3-E1 cells in vitro
Abstract
Background: It has been indicated that moderate or high dose of X-irradiation could delay fracture union and cause osteoradionecrosis, in part, mediated by its effect on proliferation and differentiation of osteoblasts. However, whether low dose irradiation (LDI) has similar roles on osteoblasts is still unknown. In this study, we investigated whether and to what extent LDI could affect the proliferation, differentiation and mineralization of osteoblasts in vitro.
Methods: The MC3T3-E1 cells were exposed to single dose of X-irradiation with 0, 0.1, 0.5, 1.0 Gy respectively. Cell proliferation, apoptosis, alkaline phosphatase (ALP) activity, and mineralization was evaluated by methylthiazoletetrazolium (MTT) and bromodeoxyuridine (BrdU) assay, flow cytometry, ALP viability kit and von Kossa staining, respectively. Osteocalcin (OCN) and core-binding factor α1 (Cbfα1) expressions were measured by real time-PCR and western blot, respectively.
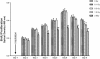
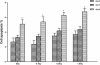
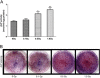
Results: The proliferation of the cells exposed to 2.0 Gy was significantly lower than those exposed to ≤1.0 Gy (p < 0.05) from Day 4 to Day 8, measured by MTT assay and BrdU incorporation. For cells exposed to ≤1.0 Gy, increasing dosages of X-irradiation had no significant effect on cell proliferation and apoptosis. Importantly, LDI of 0.5 and 1 Gy increased ALP activities and mineralized nodules of MC3T3-E1 cells. In addition, mRNA and protein expressions of OCN and Cbfα1 were also markedly increased after treatment with LDI at 0.5 and 1 Gy.
Conclusions: LDI have different effects on proliferation and differentiation of osteoblasts from those of high dose of X-irradiation, which might suggest that LDI could lead to promotion of fracture healing through enhancing the differentiation and mineralization of osteoblasts.
Figures
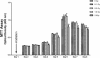
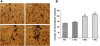
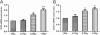
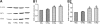
Similar articles
-
Low-dose X-ray irradiation promotes osteoblast proliferation, differentiation and fracture healing.PLoS One. 2014 Aug 4;9(8):e104016. doi: 10.1371/journal.pone.0104016. eCollection 2014. PLoS One. 2014. PMID: 25089831 Free PMC article.
-
Combined effects of γ-irradiation and cadmium exposures on osteoblasts in vitro.Environ Toxicol Pharmacol. 2012 Mar;33(2):149-57. doi: 10.1016/j.etap.2011.12.009. Epub 2011 Dec 13. Environ Toxicol Pharmacol. 2012. PMID: 22209727
-
The Effects of Photobiomodulation on MC3T3-E1 Cells via 630 nm and 810 nm Light-Emitting Diode.Med Sci Monit. 2019 Nov 19;25:8744-8752. doi: 10.12659/MSM.920396. Med Sci Monit. 2019. PMID: 31743330 Free PMC article.
-
The dual-effects of LaCl₃ on the proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 cells.Biol Trace Elem Res. 2012 Dec;150(1-3):433-40. doi: 10.1007/s12011-012-9486-6. Epub 2012 Aug 14. Biol Trace Elem Res. 2012. PMID: 22886987
-
X-ray radiation at low doses stimulates differentiation and mineralization of mouse calvarial osteoblasts.BMB Rep. 2012 Oct;45(10):571-6. doi: 10.5483/bmbrep.2012.45.10.101. BMB Rep. 2012. PMID: 23101511
Cited by
-
Diabetic HDL is dysfunctional in stimulating endothelial cell migration and proliferation due to down regulation of SR-BI expression.PLoS One. 2012;7(11):e48530. doi: 10.1371/journal.pone.0048530. Epub 2012 Nov 2. PLoS One. 2012. PMID: 23133640 Free PMC article.
-
Low-dose X-ray irradiation promotes osteoblast proliferation, differentiation and fracture healing.PLoS One. 2014 Aug 4;9(8):e104016. doi: 10.1371/journal.pone.0104016. eCollection 2014. PLoS One. 2014. PMID: 25089831 Free PMC article.
-
Identification of the mitochondrial protein ADCK2 as a therapeutic oncotarget of NSCLC.Int J Biol Sci. 2022 Oct 24;18(16):6163-6175. doi: 10.7150/ijbs.78354. eCollection 2022. Int J Biol Sci. 2022. PMID: 36439873 Free PMC article.
-
Differences in responses to X-ray exposure between osteoclast and osteoblast cells.J Radiat Res. 2017 Nov 1;58(6):791-802. doi: 10.1093/jrr/rrx026. J Radiat Res. 2017. PMID: 28541506 Free PMC article.
-
Organ-Specific Effects of Low Dose Radiation Exposure: A Comprehensive Review.Front Genet. 2020 Oct 2;11:566244. doi: 10.3389/fgene.2020.566244. eCollection 2020. Front Genet. 2020. PMID: 33133150 Free PMC article. Review.
References
-
- Mitchell MJ, Logan PM. Radiation-induced changes in bone. Radiographics. 1998;18:1125–1136. - PubMed
-
- Gebhard FT, Kraus MD, Schneider E, Liener UC, Kinzl L, Arand M. Does computer-assisted spine surgery reduce intraoperative radiation doses? Spine. 2006;31:2024–2027. doi: 10.1097/01.brs.0000229250.69369.ac. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

