An EEG-based study of discrete isometric and isotonic human lower limb muscle contractions
- PMID: 22682644
- PMCID: PMC3476535
- DOI: 10.1186/1743-0003-9-35
An EEG-based study of discrete isometric and isotonic human lower limb muscle contractions
Abstract
Background: Electroencephalography (EEG) combined with independent component analysis enables functional neuroimaging in dynamic environments including during human locomotion. This type of functional neuroimaging could be a powerful tool for neurological rehabilitation. It could enable clinicians to monitor changes in motor control related cortical dynamics associated with a therapeutic intervention, and it could facilitate noninvasive electrocortical control of devices for assisting limb movement to stimulate activity dependent plasticity. Understanding the relationship between electrocortical dynamics and muscle activity will be helpful for incorporating EEG-based functional neuroimaging into clinical practice. The goal of this study was to use independent component analysis of high-density EEG to test whether we could relate electrocortical dynamics to lower limb muscle activation in a constrained motor task. A secondary goal was to assess the trial-by-trial consistency of the electrocortical dynamics by decoding the type of muscle action.
Methods: We recorded 264-channel EEG while 8 neurologically intact subjects performed isometric and isotonic, knee and ankle exercises at two different effort levels. Adaptive mixture independent component analysis (AMICA) parsed EEG into models of underlying source signals. We generated spectrograms for all electrocortical source signals and used a naïve Bayesian classifier to decode exercise type from trial-by-trial time-frequency data.
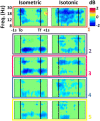
Results: AMICA captured different electrocortical source distributions for ankle and knee tasks. The fit of single-trial EEG to these models distinguished knee from ankle tasks with 80% accuracy. Electrocortical spectral modulations in the supplementary motor area were significantly different for isometric and isotonic tasks (p < 0.05). Isometric contractions elicited an event related desynchronization (ERD) in the α-band (8-12 Hz) and β-band (12-30 Hz) at joint torque onset and offset. Isotonic contractions elicited a sustained α- and β-band ERD throughout the trial. Classifiers based on supplementary motor area sources achieved a 4-way classification accuracy of 69% while classifiers based on electrocortical sources in multiple brain regions achieved a 4-way classification accuracy of 87%.
Conclusions: Independent component analysis of EEG reveals unique spatial and spectro-temporal electrocortical properties for different lower limb motor tasks. Using a broad distribution of electrocortical signals may improve classification of human lower limb movements from single-trial EEG.
Figures





References
-
- Weiller C. Imaging recovery from stroke. Exp Brain Res. 1998;123(1–2):13–17. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

