Interplay of antibiotics and bacterial inoculum on suture-associated biofilms
- PMID: 22682712
- PMCID: PMC3498097
- DOI: 10.1016/j.jss.2012.04.040
Interplay of antibiotics and bacterial inoculum on suture-associated biofilms
Abstract
Background: Biofilms are often antibiotic resistant, and it is unclear if prophylactic antibiotics can effectively prevent biofilm formation. Experiments were designed to test the ability of high (bactericidal) concentrations of ampicillin (AMP), vancomycin (VAN), and oxacillin (OXA) to prevent formation of suture-associated biofilms initiated with low (10(4)) and high (10(7)) numbers of Staphylococcus aureus.
Materials and methods: S. aureus biofilms were cultivated overnight on silk suture incubated in biofilm growth medium supplemented with bactericidal concentrations of AMP, VAN, or OXA. Standard microbiological methods were used to quantify total numbers of viable suture-associated S. aureus. Crystal violet staining followed by spectroscopy was used to quantify biofilm biomass, which includes bacterial cells plus matrix components. To observe the effects of antibiotics on the microscopic appearance of biofilm formation, biofilms were cultivated on glass slides, then stained with fluorescent dyes, and observed by confocal microscopy.
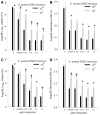
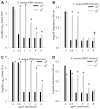
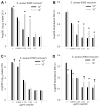
Results: In the presence of a relatively low inoculum (10(4)) of S. aureus cells, bactericidal concentrations of AMP, VAN, or OXA were effective in preventing development of suture-associated biofilms. However, similar concentrations of these antibiotics were typically ineffective in preventing biofilm development on sutures inoculated with 10(7)S. aureus, a concentration relevant to contaminated skin. Confocal microscopy confirmed that bactericidal concentrations of AMP, VAN, or OXA inhibited, but did not prevent, development of S. aureus biofilms.
Conclusion: Bactericidal concentrations of AMP, VAN, or OXA inhibited formation of suture-associated biofilms initiated with low numbers (10(4)), but not high numbers (10(7)), of S. aureus cells.
Copyright © 2012. Published by Elsevier Inc.
Figures

Similar articles
-
Gentamicin promotes Staphylococcus aureus biofilms on silk suture.J Surg Res. 2011 Oct;170(2):302-8. doi: 10.1016/j.jss.2011.06.011. Epub 2011 Jul 7. J Surg Res. 2011. PMID: 21816417 Free PMC article.
-
Relation between antibiotic susceptibility and ultrastructure of Staphylococcus aureus biofilms on surgical suture.Surg Infect (Larchmt). 2011 Aug;12(4):297-305. doi: 10.1089/sur.2010.104. Surg Infect (Larchmt). 2011. PMID: 21859333 Free PMC article.
-
Aminoglycoside inhibition of Staphylococcus aureus biofilm formation is nutrient dependent.J Med Microbiol. 2014 Jun;63(Pt 6):861-869. doi: 10.1099/jmm.0.068130-0. Epub 2014 Apr 2. J Med Microbiol. 2014. PMID: 24696518 Free PMC article.
-
Staphylococcus aureus biofilm as a target for single or repeated doses of oxacillin, vancomycin, linezolid and/or lysostaphin.Folia Microbiol (Praha). 2006;51(5):381-6. doi: 10.1007/BF02931580. Folia Microbiol (Praha). 2006. PMID: 17176756
-
Fighting Staphylococcus aureus Biofilms with Monoclonal Antibodies.Trends Microbiol. 2019 Apr;27(4):303-322. doi: 10.1016/j.tim.2018.12.009. Epub 2019 Jan 19. Trends Microbiol. 2019. PMID: 30665698 Free PMC article. Review.
Cited by
-
Use of affinity allows anti-inflammatory and anti-microbial dual release that matches suture wound resolution.J Biomed Mater Res A. 2019 Jul;107(7):1434-1442. doi: 10.1002/jbm.a.36658. Epub 2019 Mar 20. J Biomed Mater Res A. 2019. PMID: 30771234 Free PMC article.
-
The incidence of VP shunt infection in a middle-income nation: a retrospective analysis of a pediatric population.Front Surg. 2023 Dec 20;10:1304105. doi: 10.3389/fsurg.2023.1304105. eCollection 2023. Front Surg. 2023. PMID: 38174212 Free PMC article.
-
C1q is elevated during chronic Staphylococcus epidermidis central nervous system catheter infection.Front Immunol. 2024 May 31;15:1342467. doi: 10.3389/fimmu.2024.1342467. eCollection 2024. Front Immunol. 2024. PMID: 38881889 Free PMC article.
-
Non-aqueous glycerol monolaurate gel exhibits antibacterial and anti-biofilm activity against Gram-positive and Gram-negative pathogens.PLoS One. 2015 Mar 23;10(3):e0120280. doi: 10.1371/journal.pone.0120280. eCollection 2015. PLoS One. 2015. PMID: 25799455 Free PMC article.
-
The Natural Surfactant Glycerol Monolaurate Significantly Reduces Development of Staphylococcus aureus and Enterococcus faecalis Biofilms.Surg Infect (Larchmt). 2015 Oct;16(5):538-42. doi: 10.1089/sur.2014.162. Epub 2015 Jun 25. Surg Infect (Larchmt). 2015. PMID: 26110557 Free PMC article.
References
-
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34. - PubMed
-
- Cogan NG, Gunn JS, Wozniak DJ. Biofilms and infectious diseases: biology to mathematics and back again. FEMS Microbiol Lett. 2011;322:1. - PubMed
-
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034. - PubMed
-
- Cos P, Tote K, Horemans T, et al. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr Pharm Des. 2010;16:2279. - PubMed
-
- Monds RD, O’Toole GA. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 2009;17:73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

