The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development
- PMID: 22683125
- PMCID: PMC3389310
- DOI: 10.1016/j.immuni.2012.05.016
The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development
Abstract
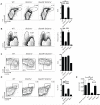
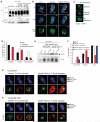
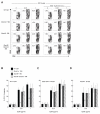
Humans and mice deficient in the adaptor protein SAP (Sh2d1a) have a major defect in humoral immunity, resulting from a lack of T cell help for B cells. The role of SAP in this process is incompletely understood. We found that deletion of receptor Ly108 (Slamf6) in CD4(+) T cells reversed the Sh2d1a(-/-) phenotype, eliminating the SAP requirement for germinal centers. This potent negative signaling by Ly108 required immunotyrosine switch motifs (ITSMs) and SHP-1 recruitment, resulting in high amounts of SHP-1 at the T cell:B cell synapse, limiting T cell:B cell adhesion. Ly108-negative signaling was important not only in CD4(+) T cells; we found that NKT cell differentiation was substantially restored in Slamf6(-/-)Sh2d1a(-/-) mice. The ability of SAP to regulate both positive and negative signals in T cells can explain the severity of SAP deficiency and highlights the importance of SAP and SHP-1 competition for Ly108 ITSM binding as a rheostat for the magnitude of T cell help to B cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
SAP signaling: a dual mechanism of action.Immunity. 2012 Jun 29;36(6):899-901. doi: 10.1016/j.immuni.2012.06.002. Immunity. 2012. PMID: 22749348
References
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annual review of immunology. 2007;25:297–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

