Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways
- PMID: 22683312
- PMCID: PMC3409440
- DOI: 10.1016/j.ajpath.2012.04.024
Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways
Abstract
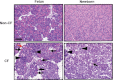
Pancreatic disease has onset in utero in humans with cystic fibrosis (CF), and progresses over time to complete destruction of the organ. The exact mechanisms leading to pancreatic damage in CF are incompletely understood. Inflammatory cells are present in the pancreas of newborn pigs with CF (CF pigs) and humans, which suggests that inflammation may have a role in the destructive process. We wondered whether tissue inflammation and genes associated with inflammatory pathways were increased in the pancreas of fetal CF pigs [83 to 90 days gestation (normal pig gestation is ~114 days)] and newborn pigs. Compared with fetal pigs without CF (non-CF pigs), in fetal CF pigs, the pancreas exhibited patchy inflammation and acinar atrophy, with progression in distribution and severity in neonatal CF pigs. Large-scale transcript profiling revealed that the pancreas in fetal and newborn CF pigs exhibited significantly increased expression of proinflammatory, complement cascade, and profibrotic genes when compared with fetal and newborn non-CF pigs. Acinar cells exhibited increased apoptosis in the pancreas of fetal and newborn CF pigs. α-Smooth muscle actin and transforming growth factor β1 were increased in both fetal and newborn CF pig pancreas, suggesting activation of profibrotic pathways. Cell proliferation and mucous cell metaplasia were detected in newborn, but not fetal, CF pigs, indicating that they were not an initiator of pathogenesis but a response. Proinflammatory, complement cascade, proapoptotic, and profibrotic pathways are activated in CF pig pancreas, and likely contribute to the destructive process.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Welsh M.J., Ramsey B.W., Accurso F.J., Cutting G.R. Cystic fibrosis. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. ed 8. McGraw-Hill; New York: 2001. pp. 5121–5188.
-
- Borowitz D., Durie P.R., Clarke L.L., Werlin S.L., Taylor C.J., Semler J., De Lisle R.C., Lewindon P., Lichtman S.M., Sinaasappel M., Baker R.D., Baker S.S., Verkade H.J., Lowe M.E., Stallings V.A., Janghorbani M., Butler R., Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–285. - PubMed
-
- Ooi C.Y., Dorfman R., Cipolli M., Gonska T., Castellani C., Keenan K., Freedman S.D., Zielenski J., Berthiaume Y., Corey M., Schibli S., Tullis E., Durie P.R. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140:153–161. - PubMed
-
- Dodge J.A., Lewis P.A., Stanton M., Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J. 2007;29:522–526. - PubMed
-
- Kopelman H., Durie P., Gaskin K., Weizman Z., Forstner G. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med. 1985;312:329–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

