Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation
- PMID: 22683614
- PMCID: PMC3467354
- DOI: 10.1016/j.bbmt.2012.05.015
Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation
Abstract
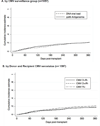
Cytomegalovirus (CMV) surveillance and preemptive therapy is the most commonly used strategy for CMV disease prevention in hematopoietic cell transplantation recipients. In 2007, we introduced a CMV prevention strategy for patients at risk for CMV disease using quantitative PCR surveillance, with treatment thresholds determined by patient risk factors. Patients (N = 367) received preemptive therapy either at a plasma viral load of ≥500 copies/mL, at ≥100 copies/mL if receiving ≥1 mg/kg of prednisone or anti-T cell therapies, or if a ≥5-fold viral load increase from baseline was detected. Compared with patients before 2007 undergoing antigenemia-based surveillance (n = 690) with preemptive therapy initiated for any positive level, the risk-adapted PCR-based strategy resulted in similar use of antiviral agents, and similar risks of CMV disease, toxicity, and nonrelapse mortality in multivariable models. The cumulative incidence of CMV disease by day 100 was 5.2% in the PCR group compared with 5.8% in the antigenemia group (1 year: 9.1% PCR vs 9.6% antigenemia). Breakthrough CMV disease in the PCR group was predominantly in the gastrointestinal (GI) tract (15 of 19 cases; 79%). However, unlike CMV pneumonia, CMV GI disease was not associated with increased nonrelapse mortality (adjusted hazard ratio, 1.19; P = .70 [GI disease] vs 8.18; P < .001 [pneumonia]). Thus, the transition to a preemptive therapy strategy based on CMV viral load and host risk factors successfully prevented CMV disease without increasing the proportion of patients receiving preemptive therapy and attributable toxicity. Breakthrough disease in PCR-based preemptive therapy occurs at a low incidence and presents primarily as GI disease, which is more likely to be responsive to antiviral therapy.
Copyright © 2012 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Boeckh M, Gooley T, Myerson D, Cunningham T, Schoch G, Bowden R. Cytomegalovirus pp65 antigenemia guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063. - PubMed
-
- Fraser GAM. Walker II and Canadian Blood and Marrow Transplant Group. Cytomegalovirus prophylaxis and treatment after hematopoietic stem cell transplantation in Canada: a description of current practices and comparison with Centers for Disease Control/Infectious Diseases Society of America/American Society for Blood and Marrow Transplantation guideline recommendations. Biol Blood Marrow Transplant. 2004;10:287–297. - PubMed
-
- Boeckh M, Gallez-Hawkins GM, Myerson D, Zaia JA, Bowden RA. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation. 1997;64:108–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA18029/CA/NCI NIH HHS/United States
- HL36444/HL/NHLBI NIH HHS/United States
- CA15704/CA/NCI NIH HHS/United States
- K23 HL096831/HL/NHLBI NIH HHS/United States
- NHLBI K23HL096831,/PHS HHS/United States
- NIAID K24HL093294/HL/NHLBI NIH HHS/United States
- K24 HL093294/HL/NHLBI NIH HHS/United States
- P01 CA018029/CA/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- CA78902/CA/NCI NIH HHS/United States
- NIAID K24AI-071113/PHS HHS/United States
- P01 HL036444/HL/NHLBI NIH HHS/United States
- HL093294/HL/NHLBI NIH HHS/United States
- P01 CA078902/CA/NCI NIH HHS/United States
- K24 AI071113/AI/NIAID NIH HHS/United States