Calcium-channel number critically influences synaptic strength and plasticity at the active zone
- PMID: 22683682
- PMCID: PMC4891200
- DOI: 10.1038/nn.3129
Calcium-channel number critically influences synaptic strength and plasticity at the active zone
Abstract
How synaptic-vesicle release is controlled at the basic release structure, the active zone, is poorly understood. By performing cell-attached current and capacitance recordings predominantly at single active zones in rat calyces, we found that single active zones contained 5-218 (mean, 42) calcium channels and 1-10 (mean, 5) readily releasable vesicles (RRVs) and released 0-5 vesicles during a 2-ms depolarization. Large variation in the number of calcium channels caused wide variation in release strength (measured during a 2-ms depolarization) by regulating the RRV release probability (P(RRV)) and the RRV number. Consequently, an action potential opened ∼1-35 (mean, ∼7) channels, resulting in different release probabilities at different active zones. As the number of calcium-channels determined P(RRV), it critically influenced whether subsequent release would be facilitated or depressed. Regulating calcium channel density at active zones may thus be a major mechanism to yield synapses with different release properties and plasticity. These findings may explain large differences reported at synapses regarding release strength (release of 0, 1 or multiple vesicles), P(RRV), short-term plasticity, calcium transients and the requisite calcium-channel number for triggering release.
Figures
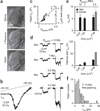

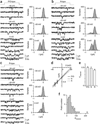



Comment in
-
In the zone: presynaptic function at high res.Nat Neurosci. 2012 Jun 26;15(7):928-9. doi: 10.1038/nn.3149. Nat Neurosci. 2012. PMID: 22735510 No abstract available.
References
-
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. - PubMed
-
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. - PubMed
-
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. - PubMed
-
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

