Release probability of hippocampal glutamatergic terminals scales with the size of the active zone
- PMID: 22683683
- PMCID: PMC3386897
- DOI: 10.1038/nn.3137
Release probability of hippocampal glutamatergic terminals scales with the size of the active zone
Erratum in
-
Corrigendum: Release probability of hippocampal glutamatergic terminals scales with the size of the active zone.Nat Neurosci. 2016 Jan;19(1):172. doi: 10.1038/nn0116-172. Nat Neurosci. 2016. PMID: 26713747 No abstract available.
Abstract
Cortical synapses have structural, molecular and functional heterogeneity; our knowledge regarding the relationship between their ultrastructural and functional parameters is still fragmented. Here we asked how the neurotransmitter release probability and presynaptic [Ca(2+)] transients relate to the ultrastructure of rat hippocampal glutamatergic axon terminals. Two-photon Ca(2+) imaging-derived optical quantal analysis and correlated electron microscopic reconstructions revealed a tight correlation between the release probability and the active-zone area. Peak amplitude of [Ca(2+)] transients in single boutons also positively correlated with the active-zone area. Freeze-fracture immunogold labeling revealed that the voltage-gated calcium channel subunit Cav2.1 and the presynaptic protein Rim1/2 are confined to the active zone and their numbers scale linearly with the active-zone area. Gold particles labeling Cav2.1 were nonrandomly distributed in the active zones. Our results demonstrate that the numbers of several active-zone proteins, including presynaptic calcium channels, as well as the number of docked vesicles and the release probability, scale linearly with the active-zone area.
Figures
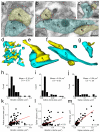
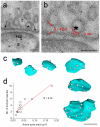
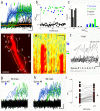
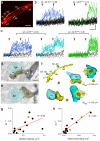
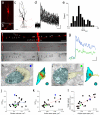

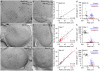

Comment in
-
In the zone: presynaptic function at high res.Nat Neurosci. 2012 Jun 26;15(7):928-9. doi: 10.1038/nn.3149. Nat Neurosci. 2012. PMID: 22735510 No abstract available.
References
-
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat. Rev. Neurosci. 2002;3:497–516. - PubMed
-
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat. Neurosci. 1999;2:618–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

