Vagal nerve stimulation blocks peritoneal macrophage inflammatory responsiveness after severe burn injury
- PMID: 22683732
- PMCID: PMC3422402
- DOI: 10.1097/SHK.0b013e31825f5fb2
Vagal nerve stimulation blocks peritoneal macrophage inflammatory responsiveness after severe burn injury
Abstract
Large surface area burn injuries lead to activation of the innate immune system, which can be blocked by parasympathetic inputs mediated by the vagus nerve. We hypothesized that vagal nerve stimulation (VNS) would alter the inflammatory response of peritoneal macrophages after severe burn injury. Male BALB/c mice underwent right cervical VNS before 30% total body surface area steam burn and were compared with animals subjected to burn alone. Peritoneal macrophages were harvested at several time points following injury and exposed to lipopolysaccharide (LPS) in culture conditions. The inflammatory response of peritoneal macrophages was measured by analyzing changes in nuclear factor κB p65 phosphorylation using flow cytometry. We found that peritoneal macrophages isolated from mice subjected to burn injury were hyperresponsive to LPS challenge, suggesting burn-induced macrophage activation. We identified a protective role for VNS in blocking peritoneal macrophage activation. Analysis of the phosphorylation state of nuclear factor κB pathway mediator, p65 Rel A, revealed a VNS-mediated reduction in p65 phosphorylation levels after exposure to LPS compared with burn alone. In combination, these studies suggest VNS mediates the inflammatory response in peritoneal macrophages by affecting the set point of LPS responsiveness.
Figures



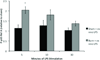


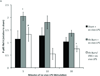
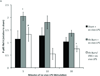
References
-
- Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. - PMC - PubMed
-
- Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. - PubMed
-
- Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157(6):2577–2585. - PubMed
-
- Ogle CK, Mao JX, Wu JZ, Ogle JD, Alexander JW. The 1994 Lindberg Award. The production of tumor necrosis factor, interleuki1-1, interleukin-6, and prostaglandin E2 by isolated enterocytes and gut macrophages: effect of lipopolysaccharide and thermal injury. J Burn Care Rehabil. 1994;15(6):470–477. - PubMed
-
- Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76(3):545–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

