In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1
- PMID: 22683847
- PMCID: PMC3425822
- DOI: 10.1096/fj.12-208975
In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1
Abstract
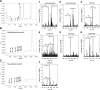
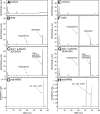
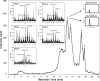
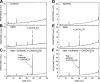
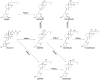
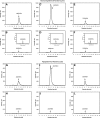
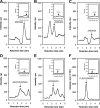
We define previously unrecognized in vivo pathways of vitamin D(3) (D3) metabolism generating novel D3-hydroxyderivatives different from 25-hydroxyvitamin D(3) [25(OH)D3] and 1,25(OH)(2)D3. Their novel products include 20-hydroxyvitamin D(3) [20(OH)D3], 22(OH)D3, 20,23(OH)(2)D3, 20,22(OH)(2)D3, 1,20(OH)(2)D3, 1,20,23(OH)(3)D3, and 17,20,23(OH)(3)D3 and were produced by placenta, adrenal glands, and epidermal keratinocytes. We detected the predominant metabolite [20(OH)D3] in human serum with a relative concentration ∼20 times lower than 25(OH)D3. Use of inhibitors and studies performed with isolated mitochondria and purified enzymes demonstrated involvement of the steroidogenic enzyme cytochrome P450scc (CYP11A1) as well as CYP27B1 (1α-hydroxylase). In placenta and adrenal glands with high CYP11A1 expression, the predominant pathway was D3 → 20(OH)D3 → 20,23(OH)(2)D3 → 17,20,23(OH)(3)D3 with further 1α-hydroxylation, and minor pathways were D3 → 25(OH)D3 → 1,25(OH)(2)D3 and D3 → 22(OH)D3 → 20,22(OH)(2)D3. In epidermal keratinocytes, we observed higher proportions of 22(OH)D3 and 20,22(OH)(2)D3. We also detected endogenous production of 20(OH)D3, 22(OH) D3, 20,23(OH)(2)D3, 20,22(OH)(2)D3, and 17,20,23(OH)(3)D3 by immortalized human keratinocytes. Thus, we provide in vivo evidence for novel pathways of D3 metabolism initiated by CYP11A1, with the product profile showing organ/cell type specificity and being modified by CYP27B1 activity. These findings define the pathway intermediates as natural products/endogenous bioregulators and break the current dogma that vitamin D is solely activated through the sequence D3 → 25(OH)D3 → 1,25(OH)(2)D3.
Figures





References
-
- Holick M. F. (2003) Vitamin D: A millennium perspective. J. Cell. Biochem. 88, 296–307 - PubMed
-
- Holick M. F. (2007) Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 - PubMed
-
- Plum L. A., DeLuca H. F. (2010) Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 9, 941–955 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

