Periostin promotes chronic allergic inflammation in response to Th2 cytokines
- PMID: 22684102
- PMCID: PMC3386810
- DOI: 10.1172/JCI58978
Periostin promotes chronic allergic inflammation in response to Th2 cytokines
Abstract
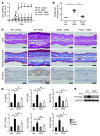
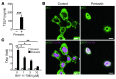
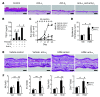
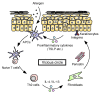
Allergic inflammation triggered by exposure of an allergen frequently leads to the onset of chronic inflammatory diseases such as atopic dermatitis (AD) and bronchial asthma. The mechanisms underlying chronicity in allergic inflammation remain unresolved. Periostin, a recently characterized matricellular protein, interacts with several cell surface integrin molecules, providing signals for tissue development and remodeling. Here we show that periostin is a critical mediator for the amplification and persistence of allergic inflammation using a mouse model of skin inflammation. Th2 cytokines IL-4 and IL-13 stimulated fibroblasts to produce periostin, which interacted with αv integrin, a functional periostin receptor on keratinocytes, inducing production of proinflammatory cytokines, which consequently accelerated Th2-type immune responses. Accordingly, inhibition of periostin or αv integrin prevented the development or progression of allergen-induced skin inflammation. Thus, periostin sets up a vicious circle that links Th2-type immune responses to keratinocyte activation and plays a critical role in the amplification and chronicity of allergic skin inflammation.
Figures



Similar articles
-
Periostin contributes to the pathogenesis of atopic dermatitis by inducing TSLP production from keratinocytes.Allergol Int. 2012 Dec;61(4):563-72. doi: 10.2332/allergolint.10-OA-0297. Epub 2012 Aug 25. Allergol Int. 2012. PMID: 22918211
-
The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation.Allergy. 2018 Sep;73(9):1881-1891. doi: 10.1111/all.13437. Allergy. 2018. PMID: 29528494
-
Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-cell Lymphoma.Cancer Res. 2016 Nov 1;76(21):6241-6252. doi: 10.1158/0008-5472.CAN-16-0992. Epub 2016 Sep 6. Cancer Res. 2016. PMID: 27634769
-
Periostin in allergic inflammation.Allergol Int. 2014 Jun;63(2):143-51. doi: 10.2332/allergolint.13-RAI-0663. Epub 2014 Mar 25. Allergol Int. 2014. PMID: 24662806 Review.
-
Periostin in Allergic Inflammation.Allergol Int. 2014;63(2):143-151. doi: 10.2332/allergolint.13-RAI-0663. Epub 2015 Feb 27. Allergol Int. 2014. PMID: 28942959 Review.
Cited by
-
Euphorbia hirta Leaf Ethanol Extract Suppresses TNF-α/IFN-γ-Induced Inflammatory Response via Down-Regulating JNK or STAT1/3 Pathways in Human Keratinocytes.Life (Basel). 2022 Apr 15;12(4):589. doi: 10.3390/life12040589. Life (Basel). 2022. PMID: 35455080 Free PMC article.
-
Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement.Am J Respir Crit Care Med. 2018 Dec 15;198(12):e116-e136. doi: 10.1164/rccm.201810-1895ST. Am J Respir Crit Care Med. 2018. PMID: 30640517 Free PMC article.
-
Osteopontin and Regulatory T Cells in Effector Phase of Allergic Contact Dermatitis.J Clin Med. 2023 Feb 9;12(4):1397. doi: 10.3390/jcm12041397. J Clin Med. 2023. PMID: 36835932 Free PMC article.
-
The Multiple Roles of Periostin in Non-Neoplastic Disease.Cells. 2022 Dec 22;12(1):50. doi: 10.3390/cells12010050. Cells. 2022. PMID: 36611844 Free PMC article. Review.
-
Potential Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in the Management of Atopic Dermatitis.Molecules. 2022 Jul 21;27(14):4660. doi: 10.3390/molecules27144660. Molecules. 2022. PMID: 35889539 Free PMC article. Review.
References
-
- Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009;102:135–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

