Engineering domain fusion chimeras from I-OnuI family LAGLIDADG homing endonucleases
- PMID: 22684507
- PMCID: PMC3439895
- DOI: 10.1093/nar/gks502
Engineering domain fusion chimeras from I-OnuI family LAGLIDADG homing endonucleases
Abstract
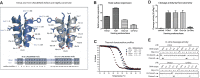
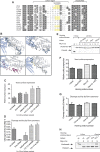
Although engineered LAGLIDADG homing endonucleases (LHEs) are finding increasing applications in biotechnology, their generation remains a challenging, industrial-scale process. As new single-chain LAGLIDADG nuclease scaffolds are identified, however, an alternative paradigm is emerging: identification of an LHE scaffold whose native cleavage site is a close match to a desired target sequence, followed by small-scale engineering to modestly refine recognition specificity. The application of this paradigm could be accelerated if methods were available for fusing N- and C-terminal domains from newly identified LHEs into chimeric enzymes with hybrid cleavage sites. Here we have analyzed the structural requirements for fusion of domains extracted from six single-chain I-OnuI family LHEs, spanning 40-70% amino acid identity. Our analyses demonstrate that both the LAGLIDADG helical interface residues and the linker peptide composition have important effects on the stability and activity of chimeric enzymes. Using a simple domain fusion method in which linker peptide residues predicted to contact their respective domains are retained, and in which limited variation is introduced into the LAGLIDADG helix and nearby interface residues, catalytically active enzymes were recoverable for ≈ 70% of domain chimeras. This method will be useful for creating large numbers of chimeric LHEs for genome engineering applications.
Figures



References
-
- Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008;9:297–308. - PubMed
-
- Karran P. DNA double strand break repair in mammalian cells. Curr. Opin. Genet. Dev. 2000;10:144–150. - PubMed
-
- McCammon JM, Amacher SL. Using zinc finger nucleases for efficient and heritable gene disruption in zebrafish. Methods Mol. Biol. 2010;649:281–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

