Control of alternative splicing by forskolin through hnRNP K during neuronal differentiation
- PMID: 22684629
- PMCID: PMC3439897
- DOI: 10.1093/nar/gks504
Control of alternative splicing by forskolin through hnRNP K during neuronal differentiation
Abstract
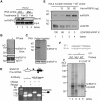
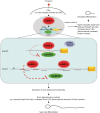
The molecular basis of cell signal-regulated alternative splicing at the 3' splice site remains largely unknown. We isolated a protein kinase A-responsive ribonucleic acid (RNA) element from a 3' splice site of the synaptosomal-associated protein 25 (Snap25) gene for forskolin-inhibited splicing during neuronal differentiation of rat pheochromocytoma PC12 cells. The element binds specifically to heterogeneous nuclear ribonucleo protein (hnRNP) K in a phosphatase-sensitive way, which directly competes with the U2 auxiliary factor U2AF65, an essential component of early spliceosomes. Transcripts with similarly localized hnRNP K target motifs upstream of alternative exons are enriched in genes often associated with neurological diseases. We show that such motifs upstream of the Runx1 exon 6 also bind hnRNP K, and importantly, hnRNP K is required for forskolin-induced repression of the exon. Interestingly, this exon encodes the peptide domain that determines the switch of the transcriptional repressor/activator activity of Runx1, a change known to be critical in specifying neuron lineages. Consistent with an important role of the target genes in neurons, knocking down hnRNP K severely disrupts forskolin-induced neurite growth. Thus, through hnRNP K, the neuronal differentiation stimulus forskolin targets a critical 3' splice site component of the splicing machinery to control alternative splicing of crucial genes. This also provides a regulated direct competitor of U2AF65 for cell signal control of 3' splice site usage.
Figures





References
-
- Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 2001;17:100–107. - PubMed
-
- Maniatis T, Tasic B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature. 2002;418:236–243. - PubMed
-
- Black DL. Mechanisms of alternative pre-messenger rna splicing. Annu. Rev. Biochem. 2003;72:291–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

