Self-sterility in flowering plants: preventing self-fertilization increases family diversification rates
- PMID: 22684683
- PMCID: PMC3400452
- DOI: 10.1093/aob/mcs124
Self-sterility in flowering plants: preventing self-fertilization increases family diversification rates
Erratum in
- Ann Bot. 2012 Oct;110(5):1079-81
Abstract
Background and scope: New data are presented on the distribution and frequency of self-sterility (SS) - predominantly pre-zygotic self-incompatibility (SI) systems - in flowering plants and the hypothesis is tested that families with self-sterile taxa have higher net diversification rates (DRs) than those with exclusively self-compatible taxa using both absolute and relative rate tests.
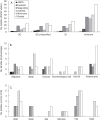
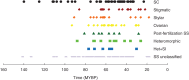
Key results: Three major forms of SI systems (where pollen is rejected at the stigmatic, stylar or ovarian interface) are found to occur in the oldest families of flowering plants, with times of divergence >100 million years before the present (mybp), while post-fertilization SS and heterostyly appear in families with crown ages of 81 and 87 mybp, respectively. It is also founnd that many (22) angiosperm families exhibit >1 SI phenotype and that the distribution of different types of SS does not show strong phylogenetic clustering, collectively suggesting that SS and SI systems have evolved repeatedly de novo in angiosperm history. Families bearing self-sterile taxa have higher absolute DRs using all available calibrations of the angiosperm tree, and this affect is caused mostly by the high DR of families with homomorphic SI systems (in particular stigmatic SI) or those in which multiple SS/SI phenotypes have been observed (polymorphic). Lastly, using sister comparisons, it is further demonstrated that in 29 of 38 sister pairs (including 95 families), the self-sterile sister group had higher species richness and DR than its self-compatible sister based on either the total number of taxa in the clade with SS or only the estimated fraction to harbour SS based on literature surveys.
Conclusions: Collectively, these analyses point to the importance of SS, particularly pre-zygotic SI in the evolution of flowering plants.
Figures







Similar articles
-
The distribution of self-incompatibility systems in angiosperms: the relationship between mating system diversity, life span, growth habit and latitude in a changing global environment.Ann Bot. 2025 Feb 8;135(1-2):25-42. doi: 10.1093/aob/mcae056. Ann Bot. 2025. PMID: 38716780 Free PMC article.
-
Plant mating systems: self-incompatibility and evolutionary transitions to self-fertility in the mustard family.Curr Opin Genet Dev. 2017 Dec;47:54-60. doi: 10.1016/j.gde.2017.08.005. Epub 2017 Sep 13. Curr Opin Genet Dev. 2017. PMID: 28915488 Review.
-
How Have Self-Incompatibility Haplotypes Diversified? Generation of New Haplotypes during the Evolution of Self-Incompatibility from Self-Compatibility.Am Nat. 2016 Aug;188(2):163-74. doi: 10.1086/687110. Epub 2016 Jun 2. Am Nat. 2016. PMID: 27420782
-
Self-incompatibility in flowering plants: the Brassica model.C R Acad Sci III. 2001 Jun;324(6):537-42. doi: 10.1016/s0764-4469(01)01323-3. C R Acad Sci III. 2001. PMID: 11455876 Review.
-
Absolute diversification rates in angiosperm clades.Evolution. 2001 Sep;55(9):1762-80. doi: 10.1111/j.0014-3820.2001.tb00826.x. Evolution. 2001. PMID: 11681732
Cited by
-
Assortative mating and self-fertilization differ in their contributions to reinforcement, cascade speciation, and diversification.Curr Zool. 2016 Apr;62(2):169-181. doi: 10.1093/cz/zow004. Epub 2016 Feb 29. Curr Zool. 2016. PMID: 29491904 Free PMC article.
-
Elevated transcription of transposable elements is accompanied by het-siRNA-driven de novo DNA methylation in grapevine embryogenic callus.BMC Genomics. 2021 Sep 20;22(1):676. doi: 10.1186/s12864-021-07973-9. BMC Genomics. 2021. PMID: 34544372 Free PMC article.
-
Evolution of self-incompatibility in the Brassicaceae: Lessons from a textbook example of natural selection.Evol Appl. 2020 Mar 3;13(6):1279-1297. doi: 10.1111/eva.12933. eCollection 2020 Jul. Evol Appl. 2020. PMID: 32684959 Free PMC article.
-
Convergent evolutionary patterns of heterostyly across angiosperms support the pollination-precision hypothesis.Nat Commun. 2024 Feb 9;15(1):1237. doi: 10.1038/s41467-024-45118-0. Nat Commun. 2024. PMID: 38336937 Free PMC article.
-
Heterostyly accelerates diversification via reduced extinction in primroses.Proc Biol Sci. 2014 Apr 23;281(1784):20140075. doi: 10.1098/rspb.2014.0075. Print 2014 Jun 7. Proc Biol Sci. 2014. PMID: 24759859 Free PMC article.
References
-
- Allen AM, Hiscock SJ. Evolution and phylogeny of self-incompatibility systems in angiosperms. In: Franklin-Tong VE, editor. Self-incompatibility in flowering plants. Berlin: Springer; 2008.
-
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121.
-
- Baker HG. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution. 1955;9:347–349.
-
- Baker HG. The evolution, functioning and breakdown of heteromorphic incompatibility systems.1. Plumbaginaceae. Evolution. 1966;20:349–368. - PubMed
-
- Barraclough TG, Harvey PH, Nee S. Sexual selection and taxonomic diversity in passerine birds. Proceedings of the Royal Society of London. Series B: Biological Sciences. 1995;259:211–215.

