Electron tomographic analysis of synaptic ultrastructure
- PMID: 22684938
- PMCID: PMC3856703
- DOI: 10.1002/cne.23067
Electron tomographic analysis of synaptic ultrastructure
Abstract
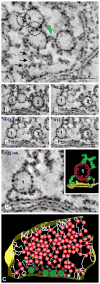
Synaptic function depends on interactions among sets of proteins that assemble into complex supramolecular machines. Molecular biology, electrophysiology, and live-cell imaging studies have provided tantalizing glimpses into the inner workings of the synapse, but fundamental questions remain regarding the functional organization of these "nano-machines." Electron tomography reveals the internal structure of synapses in three dimensions with exceptional spatial resolution. Here we report results from an electron tomographic study of axospinous synapses in neocortex and hippocampus of the adult rat, based on aldehyde-fixed material stabilized with tannic acid in lieu of postfixation with osmium tetroxide. Our results provide a new window into the structural basis of excitatory synaptic processing in the mammalian brain.
Copyright © 2012 Wiley Periodicals, Inc.
Figures







References
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
-
- Berryman MA, Porter WR, Rodewald RD, Hubbard AL. Effects of tannic acid on antigenicity and membrane contrast in ultrastructural immunocytochemistry. J Histochem Cytochem. 1992;40:845–857. - PubMed
-
- Bloom FE, Aghajanian GK. Fine structural and cyto-chemical analysis of the staining of synaptic junctions with phosphotungstic acid. J Ultrastruct Res. 1968;22:361–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

