DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice
- PMID: 22685166
- PMCID: PMC3406904
- DOI: 10.1105/tpc.112.097394
DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice
Abstract
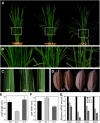
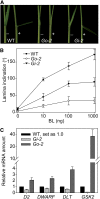
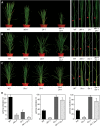
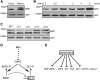
In Arabidopsis thaliana, the GSK3/SHAGGY-like kinase BRASSINOSTEROID-INSENSITIVE2 (BIN2) plays a critical role in the brassinosteroid (BR) signaling pathway by negatively regulating the activities of bri1-EMS-SUPPRESSOR1/BRASSINAZOLE-RESISTANT1 family transcription factors that regulate the expression of downstream BR-responsive genes. In this study, we analyzed the function of a rice (Oryza sativa) GSK3/SHAGGY-like kinase (GSK2), which is one of the orthologs of BIN2. Overexpression of GSK2 (Go) led to plants with typical BR loss-of-function phenotypes, and suppression of GSK2 resulted in enhanced BR signaling phenotypes. DWARF AND LOW-TILLERING (DLT) is a positive regulator that mediates several BR responses in rice. Suppression of DLT can enhance the phenotypes of BR receptor mutant d61-1, and overexpression of DLT obviously suppressed the BR loss-of-function phenotypes of both d61-1 and Go, suggesting that DLT functions downstream of GSK2 to modulate BR responses. Indeed, GSK2 can interact with DLT and phosphorylate DLT. Moreover, brassinolide treatment can induce the dephosphorylation of DLT, leading to the accumulation of dephosphorylated DLT protein. In GSK2 transgenic plants, the DLT phosphorylation level is dictated by the GSK2 level. These results demonstrate that DLT is a GSK2 substrate, further reinforcing that the BIN2/GSK2 kinase has multiple substrates that carry out various BR responses.
Figures





References
-
- Bilgin D.D., DeLucia E.H., Clough S.J. (2009). A robust plant RNA isolation method suitable for Affymetrix GeneChip analysis and quantitative real-time RT-PCR. Nat. Protoc. 4: 333–340 - PubMed
-
- Clouse S.D. (2002). Brassinosteroid signal transduction: Clarifying the pathway from ligand perception to gene expression. Mol. Cell 10: 973–982 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

